The Primary Care Companion
Information for Authors
Explore comprehensive guidelines and resources for submitting your manuscript to The Primary Care Companion for CNS Disorders, ensuring your work meets our submission standards.
The Primary Care Companion
Explore comprehensive guidelines and resources for submitting your manuscript to The Primary Care Companion for CNS Disorders, ensuring your work meets our submission standards.
Expand All
Back to top
We encourage you to submit your manuscript if it fits within the scope of The Primary Care Companion for CNS Disorders. Acceptance is contingent on favorable peer review. Our user-friendly electronic manuscript submission and peer review system allows you to submit your manuscript online securely, conveniently, and instantly. The system will request that new authors register for an account; authors who have previously submitted should use their previous account login information. Authors can track the progress of their manuscripts from submission to final decision. Assistance is available via e-mail if needed.
We are asking all clients to use electronic delivery methods to submit any information or documents needed in support of our service to you. Please do not provide information using physical mail as we may not be able to retrieve it during this time.
NOTE: This account is separate from your Psychiatrist.com and CMEInstitute.com accounts. Thus, even if you are already registered to access our sites, you will still need to establish an account in the manuscript submission system.
Founded in 1998, PCC is an international peer-reviewed online-only journal with weekly postings and 6 issues/year. PCC seeks to advance the clinical expertise of primary care physicians and other health care professionals who treat patients with mental and neurologic illnesses. PCC publishes research from disciplines such as medicine, nursing, pharmacy, and psychology, especially as it pertains to integrated delivery systems and interdisciplinary collaboration.
PCC focuses on providing information of direct clinical utility and giving a voice to clinician researchers. Practice-based research from individuals and groups with clinical expertise is particularly welcome. Pertinent manuscript types include
To submit your concisely written, appropriately referenced, and focused manuscript that fits within this scope and the word count, see the submission guidelines.
The Primary Care Companion for CNS Disorders:
Back to top
Include the following in the manuscript, prior to submission:
Include permissions from the source for the following:
At the time of manuscript submission or revision, the journal must have received a separate, completed Author Form from each individual author.
Location of a Blank Form:
The Primary Care Companion for CNS Disorders (PCC): Download Here
Author:
By signing the Author Form, the bylined author
Corresponding Author:
Submissions should have only ONE corresponding author. By signing the Author Form, the corresponding author
Submission of the Completed Form:
This form can be submitted to the journal by either:
Back to top
Persons listed as authors must have made contributions in each of these 4 areas:
All persons designated as authors should fulfill these 4 criteria, and all those who qualify should be listed as authors. All authors must have contributed sufficiently to the work to take public responsibility for the content. Acquisition of funding, collection of data, or general supervision of a research group, alone, does not justify authorship. If authorship is attributed to a group, each member must meet authorship criteria; group members who do not meet these criteria should be listed in an acknowledgment.
The corresponding author will serve on behalf of the other authors as the primary contact with the editorial office and is responsible for ensuring that the acknowledgment and direct support information are complete. This person is responsible for communicating with the other authors about revisions and final approval of the article.
Further detailed information can be found at the International Committee of Medical Journal Editors (ICMJE) website: http://www.icmje.org/.
Research for which results do not substantially overlap with previously published research by the same authors or group of authors qualifies as original research.
Manuscripts should be submitted to only one (1) journal, which is to be held in confidence with that journal until review is complete, leading to rejection, revision, or acceptance by the journal or withdrawal by the authors.
The journal will consider submissions that have been previously posted as preprints on established preprint servers that adhere to industry standards (such as bioRxiv and medRxiv).
Poster presentations and submission to a clinical trials registry do not constitute previous publication, but notation should be made in the manuscript of any presentations and clinical trials registration. If results of original research have been presented at a meeting or meetings, it does not constitute previous publication, and the manuscript may still be published for the first time with the journal. However, all information about presentations should be annotated in the manuscript.
In an endmatter footnote, include the following for each meeting:
Artificial intelligence (AI)–assisted technology does not meet the requirements for authorship and so may not be listed in the byline as an author or cited as an author. It is the responsibility of authors to ensure the accuracy or integrity of the work. If AI models or tools are used to create content or assist with writing or manuscript preparation, authors must take responsibility for the content generated and should be able to assert that there is no plagiarism in their manuscript, including content generated by the AI tool.
AI technology may be used only to improve readability and language, not to replace research tasks such as interpreting data or drawing scientific conclusions. All use of AI in the drafting of the manuscript must be disclosed. Authors who have used AI-assisted tools in the writing process must include on the title page of the manuscript a section labeled, “Use of AI-assisted technologies in the writing process.” Please use the following disclosure:
“In the writing of this manuscript, the author(s) used [name, version, manufacturer of AI tool] for [reason for use]. The author(s) have reviewed the content and take(s) full responsibility for the content of the publication.”
This policy does not apply to use of AI and AI-assisted tools in research design or methods, which should be described in the Method section.
For manuscripts that report analyses of preexisting data sets, provide details related to accessing the data set, including
This information should be inserted as a statement into the text’s Method, eg, “The original data set is available from…,” or any wording that’s appropriate, or inserted at the end of the article (just before references), eg, “Additional information: The [full proper name of database] can be found at [specific URL].”
Authors must obtain letters of permission to reproduce published material. These documents should be sent at the time of submission of the manuscript and can accompany the Author Form(s). The form(s) can be faxed to the Publications Manager at (901) 273–2752.
The World Health Organization (WHO) defines a clinical trial as “any research project that prospectively assigns human participants or groups of humans to 1 or more health-related interventions to evaluate the effects on health outcomes.”
The journal requires, as a condition of consideration for publication, registration of clinical trials in a public trials registry that meets the criteria noted in the following paragraphs. (Such registration does not constitute previous publication.)
Registration requirements are as follows:
A public trials registry must meet several criteria:
An acceptable registration must include, at minimum, the following:
For detailed information, please see “Frequently Asked Questions” of the ICMJE Recommendations (previously “Uniform Requirements for Manuscripts Submitted to Biomedical Journals”) website at http://www.icmje.org/about-icmje/faqs/clinical-trials-registration/.
The following trial registries meet the required criteria, although the journal does not advocate one particular registry:
Registry name, URL, and trial ID number must be provided in
Trial Registration: ClinicalTrials.gov identifier: NCT000123456
Trial Registration: Data used in this secondary analysis came from ClinicalTrials.gov identifier: NCT000123456
In Method section of article:
“The study was approved by the local ethics committee, written informed consent was obtained, and the study was registered at ClinicalTrials.gov (identifier: NCT000123456).”
The journal’s copyright policy applies to all manuscripts except those published open access. The journal requires the express transfer of copyright to Physicians Postgraduate Press, Inc., to protect the author(s) and Physicians Postgraduate Press, Inc., from misuse of copyrighted materials. All accepted manuscripts become the property of Physicians Postgraduate Press, Inc., and may not be published or posted online elsewhere, in part or in whole, without written permission from Physicians Postgraduate Press, Inc. (See also Terms and Conditions.)
Physicians Postgraduate Press, Inc., publisher of The Journal of Clinical Psychiatry and The Primary Care Companion for CNS Disorders, must retain exclusive and unfettered copyright of published materials either in print or electronic formats in perpetuity with the exception of materials published open access. The publisher’s copyright protection lasts the life of the author plus an additional seventy (70) years. If a signed opt-out, waiver, or other official document is not obtained, the manuscript cannot be considered for publication by Physicians Postgraduate Press, Inc.
Authors of manuscripts accepted for publication in The Journal of Clinical Psychiatry or The Primary Care Companion for CNS Disorders that report original research funded in whole or in part by an NIH grant have the journal’s permission to submit their accepted manuscript to the National Library of Medicine’s PubMed Central in accordance with the NIH Public Access Policy provided that the manuscript is made publicly available no sooner than 12 months after the official date of publication. [See National Institutes of Health (NIH)-Funded Research Articles.]
Relevant Financial Relationships:
Authors are required to provide either (1) a statement indicating relevant financial relationships involving their manuscript or (2) a statement that they have no relevant financial relationships. Conflicts should reflect activity by the author within the last 24 months and include, but are not limited to
Funding/Support:
Describe direct support in the manuscript; direct funding, support, sponsorship, or provision of materials includes salaries, equipment, supplies, etc, from organizations that may gain or lose financially through the publication of the manuscript.
Role of the Sponsor:
If the study was directly supported in any manner, then a statement on the role of the sponsor in the study must be noted, including publication of the manuscript.
Authors may include a footnote in the endmatter that acknowledges contributions of groups or persons who do not qualify for authorship. Acknowledged individuals might include those who have provided data gathering, writing, or clerical assistance; statistical or general review; or performance of special tests.
When specific individuals are acknowledged, 2 steps must be taken:
First: The Corresponding Author:
Second: The Manuscript must include the following information for each person named in the Acknowledgment:
Unpublished material includes personal communications and unpublished data such as data on file and manuscripts in preparation, submitted but not yet accepted, or in press. Each type of unpublished material must meet a specific set of criteria for it to be included in the manuscript, but the most important criterion is that written permission from the source of the unpublished material must be submitted to the journal. See the Citations section under Manuscript Components for specifics on how to handle these types of citations.
ICMJE’s Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals
Published by the International Committee of Medical Journal Editors (ICMJE), this document, formerly titled Uniform Requirements for Manuscripts Submitted to Biomedical Journals, covers ethical principles related to evaluating, improving, and publishing manuscripts, as well as technical aspects of preparing and submitting manuscripts.
CONSORT Statement
The CONSORT Statement provides guidelines for improved reporting of clinical trials through the use of a checklist and study flow diagram.
PRISMA Statement
The PRISMA Statement provides guidelines for transparent reporting of systematic reviews and meta-analyses through the use of a checklist and study flow diagram.
Back to top
Manuscripts submitted for publication in the journal that meet its scope and submission criteria are sent to expert consultants for peer review. Please see Reviewers for details about the peer review process and information on becoming a reviewer.
Manuscripts accepted for publication after peer review will be copyedited for clarity, conciseness, and conformity with journal style and returned to the corresponding author for approval. Bylined authors are responsible for all statements in their work, including changes authorized by the corresponding author.
Articles are embargoed until they are published online at PrimaryCareCompanion.com. Contact [email protected] for information about a specific “In press” article for The Primary Care Companion for CNS Disorders. (See Copyright Policy and Terms and Conditions.)
Single copies of articles or back issues, as well as larger quantities of high-quality photocopies or reprints, can be purchased from [email protected]. At the time of publication, all authors are sent notification when their manuscript publishes. Corresponding authors receive a complimentary PDF with instructions on how the PDF may and may not be used. Learn more about reprints, eprints, and permissions at this links: Reprints and Permissions. (See also Copyright Policy and Terms and Conditions.)
Physicians Postgraduate Press, Inc., owns the copyrights for the material published in its publications, The Journal of Clinical Psychiatry and The Primary Care Companion for CNS Disorders, and their associated Web sites. The information as it is presented in all forms is the property of the Company. See Reprints and Permissions for how to acquire permission to use copyrighted material.
Charges will be incurred for any changes requested after publication, with the exception of any error introduced by the publisher. Payment must be received before changes will be made to the published piece.
All materials published by Physicians Postgraduate Press, Inc., are the property of Physicians Postgraduate Press, Inc., unless otherwise stated, and are subject to all laws pertaining to copyrighted work.
Retention of copyright by Physicians Postgraduate Press, Inc., means the work may not be reproduced or transmitted in any form or by any means, including electronic, photocopying, or otherwise; distributed in any form or by any means; publicly displayed in any form including but not limited to Internet, Extranet, PowerPoint, or posters; and used for commercial, promotional, or marketing purposes, which include sale, resale, transfer, loan, license, or other forms of commercial exploitation. (See also Copyright Policy.)
Back to top
Click the link inside the table to review further details about each fee. If questions, contact the Publications Manager at [email protected]
| Type | Comments | Cost |
| Open and Free Access Options | See Article Access Options table. | See Article Access Optionstable. |
| Submission and Publication |
|
$0 |
| Extensive author-requested changes during copyediting |
|
Fee is prorated |
| Corrections/Errata | After author approval: Changes requested
after the journal receives author approval of the “for review” PDF or after the deadline for receipt of approval has expired will be considered corrections. |
$150/page |
| Requiring realignment: Journal staff make every effort to avoid realignment and avoid this charge. | + $75 per additional page that requires realignment | |
| Requiring PubMed changes or a correction to be published: If the change affects PubMed indexing information or requires a correction to be published, a fee may be charged. | + $300 | |
| Reprints | Contact [email protected] | Dependent on quantity and use |
| Permissions | Contact [email protected] | Dependent on quantity and use |
The journal does not charge submission or publication fees, eg, color printing. However, charges are associated with some aspects of article processing. See the table of Article Processing Charges and the table of Article Access Options.
Authors are given at least 2 opportunities to review their article after copyediting. They will receive:
When an article has been approved by the author to go forward with publication, or if the author fails to provide approval by the deadline and the article is moved forward without approval, the article is considered in the publication cycle and any author changes at this point will incur a charge. Charges begin at $150/page, and if realignment is required, the charge would be an additional $75/page, although every effort will be made to avoid realignment. If the change affects PubMed indexing information or requires a correction to be published, a $300 fee may also be charged. Payment must be received before changes will be made to the published article. An exception would be any error introduced by the publisher, in which case the author would not be charged.
Single copies of articles can be purchased through the website, and back issues as well as larger quantities of high-quality photocopies or reprints can be purchased by going to the Reprints and Permissions page on the website. All authors are notified when their article is published. Corresponding authors receive a complimentary PDF with instructions on how the PDF may and may not be used. Learn more about reprints, e-prints, and permissions through this link: Reprints and Permissions. (See also Copyright Policy and Terms of Use.)
All editorial material published in The Journal of Clinical Psychiatry and The Primary Care Companion for CNS Disorders or on CME Institute.com and Psychiatrist.com is the property of Physicians Postgraduate Press, Inc., unless specifically stated. See Reprints and Permissions for instructions to acquire permission to use copyrighted material. (See also Copyright Policy and Terms of Use.)
Back to top
If your research was funded by noncommercial source(s), please review the requirements of the funder regarding open access prior to submitting your manuscript.
Click the link inside the table to review further details about each fee. If questions, contact the Publications Manager at [email protected]
| Copyright | Eligibility (Based on Research Funding) |
Access Options | Cost |
|---|---|---|---|
| Green Open Access (peer-reviewed, accepted manuscript) | |||
| Authors transfer copyright to publisher | Available for articles with US National Institutes of Health, US Department of Veterans Affairs, or other government or public funding | Immediately upon publication:
(Publisher HTML and PDFs are copyrighted and may not be posted elsewhere. If your funder requires immediate self-archiving of the author accepted manuscript, contact the publisher at [email protected] for more information.) |
$0 |
| Gold Open Access (final published version of article) | |||
| CC BY or CC BY-ND license |
Available for manuscripts with funding from Plan S funding sources only | Immediately upon publication, author uploads into repository required by the funder:
(Commercial use is allowed. Publisher does not retain copyright.) |
$5,000 |
| CC BY-NC-ND license | Available for manuscripts with funding from public funders that mandate open access but are not part of the Plan S group | Immediately upon publication, the author uploads into repositories required by the funder:
(Commercial use is not allowed. Publisher does not retain copyright.) |
$5,000 |
| Free Access PDF (final published version of article) | |||
| The PCC offers authors of accepted articles and letters the opportunity to make the PDF of their article or letter freely available to readers on the journal website at PrimaryCareCompanion.com. | $500 | ||
Green open access is available for articles with US National Institutes of Health, US Department of Veterans Affairs, or other government or public funding. Immediately upon publication:
(Publisher HTML and PDFs are copyrighted and may not be posted elsewhere. If your funder requires immediate self-archiving of the author accepted manuscript, contact the publisher at [email protected] for more information.)
In accordance with the NIH Public Access Policy (http://publicaccess.nih.gov), authors of manuscripts accepted for publication in a journal that report original research funded in whole or in part by a National Institutes of Health (NIH) grant must post their research in the NIH Manuscript Submission (NIHMS) System for later deposit in the National Library of Medicine’s PubMed Central (PMC):
Submitting PDFs of the final copyedited article to PMC or NIHMS is prohibited and constitutes copyright infringement. Therefore, a PDF of the publisher’s article should not be submitted or posted. The article of record is the final version published on the journal’s website. The journal assumes no responsibility for earlier versions, which may differ substantively.
Gold Open Access is available for manuscripts with funding from Plan S funding sources. See below for the Creative Commons Copyright (CCC) licenses that may apply; the type of license will appear in the article’s copyright statement. The cost for Gold open access is $5,000. Immediately upon publication, the author uploads the PDF of the article into the repository required by the funder. Submission of payment signifies your agreement to the Terms of Use for copyrighted work (see also the Copyright Policy). Contact the Publications Manager at [email protected] to pursue this option.
PCC offers authors of accepted articles and letters the opportunity to make the PDF of their article or letter freely available to readers on PCC’s Web site, PrimaryCareCompanion.com. The charge for this service is $500. Authors are provided this option at the time of acceptance.
Of the 50,000 readers of the online PCC, all must pay a $40 fee to download an article PDF. The option of financial sponsorship allows you to provide each Web visitor unrestricted access to the PDF of your article or letter. Each sponsored article is flagged online to indicate free PDF availability. Submission of payment signifies your agreement to the Terms and Conditions for copyrighted work.
Back to top
All manuscripts, including letters to the editor, must be accompanied by an electronic cover letter. Manuscripts are reviewed with the understanding that they represent original material, have never been published before, are not under consideration for publication elsewhere, and have been approved by each author. Prior publication constitutes any form of publication other than an abstract or clinical trial registration and includes invited articles, proceedings, symposia, and book chapters. Authors should fully inform the editor in the cover letter if the submitted manuscript contains data or clinical observations that have been published or submitted for publication elsewhere, supply copies of such material, and explain the differences between the works.
Manuscripts should have margins of at least 1 in and be double-spaced throughout, including title page, abstract, text, references, tables, and legends for figures. Number pages consecutively in the upper right-hand corner, beginning with the title page. Each section should begin on a separate page, and the sections should be arranged in the following order: (1) title page, (2) abstract and key words, (3) text, and (4) references. Tables and figures should be submitted as separate file(s) from the manuscript.
For each author, provide first name, middle initial, and last name along with highest academic degree(s) and departmental and institutional affiliation, including city/state/country location.
At the bottom of the title page, list the following:
Articles:
Case Reports:
Letters to the Editor:
If you are submitting an article, you are required to include a structured abstract of about 250 words or less. The abstract must reflect the text or graphics; that is, no information should be included in the abstract that cannot be drawn from the text or graphics.
Reports of Original Data:
Objective: State the question addressed in the study.
Methods: Describe the basic study design. State the setting (eg, primary care, referral center). Explain selection of study subjects and state the system of diagnostic criteria used. Describe any interventions and include their duration and method of administration. Indicate the main outcome measure(s). Specify the dates in which data were collected (month/year to month/year).
Results: Include the key findings. Give specific data and their statistical significance, if possible (include P value if findings were significant). Subset Ns should accompany percentages if the total N is < 100.
Conclusion: Summarize the conclusions.
Clinical Trials Registration: If the article reports a clinical trial, give the trial registry name, URL, and registration number.
Systematic Reviews and Meta-Analyses:
Objective: State the primary objective of the article.
Data Sources: Describe the data sources that were searched, including dates, keywords, and constraints (eg, language limits).
Study Selection: Identify the number of studies reviewed and the criteria used for their selection.
Data Extraction: Summarize guidelines used for abstracting data and how they were applied.
Results: State the main results of the review and the methods used to obtain these results.
Conclusions: Summarize the conclusions.
Review and Case Study Combined:
Objective: State the primary objective of the article.
Data Sources: Describe the data sources that were searched, including dates, keywords, and constraints (eg, language limits). If the list of search terms is complicated and somewhat lengthy, a summary of terms can be used in the abstract.
Study Selection: Identify the number of studies retrieved and ultimately reviewed and the criteria used for their selection. By reading the abstract, the reader should be able to follow the progression of the process.
Data Extraction: Summarize guidelines used for abstracting data for the meta-analysis and how they were applied. The case-control study information under Method should be incorporated here.
Results: State the main results of the review and the methods used to obtain these results.
Conclusions: Summarize the conclusions.
Consensus Statements:
Objective: State the issue, purpose, and intended audience.
Participants: Describe how people were chosen to be participants, state the number of participants, and describe their areas of expertise. State whether meetings were open or closed.
Evidence: Describe what data sources were used and explain their selection, abstraction, and the method of their synthesis. If a formal literature review was conducted, state who wrote it and whether it was reviewed. Describe any use of unpublished data. Explain the influence of expert opinion and comments from the participants.
Consensus Process: Describe the basis for conclusions. State how consensus was achieved. Describe the writing of the consensus statement, including who wrote it, whether it was drafted before or after the group expressed its opinions, and when it was written. Explain who reviewed the statement and how revision suggestions were utilized.
Conclusions: Summarize the consensus statement, and include any important minority views.
Further details on writing informative structured abstracts can be obtained from the following:
Reports of Original Data:
The text of observational and experimental articles is usually–but not necessarily–divided into sections with the headings Introduction, Method, Results, and Discussion. Lengthy articles may need subheadings within some sections to clarify their content. Manuscripts should have a maximum length of 3,000 words (excluding abstract, tables, figures, and references).
Introduction
State the purpose of the article. Summarize the rationale for the study or observation. Give only strictly pertinent references, and do not review the subject extensively. Do not include data or conclusions from the work being reported.
Methods
Describe your selection of the observational or experimental subjects (including controls) clearly, including eligibility. Identify the methods, apparatus (manufacturer’s name and city/state/country location in parentheses), and procedures in sufficient detail to allow other workers to reproduce the results. Give references to established methods, including statistical methods (see below). Include references for all assessment tools, including scales, used in the study. Describe new or modified methods, give reasons for using them, and evaluate their limitations. Identify precisely all drugs and chemicals used, including generic name(s), dose(s), and route(s) of administration. Specify the dates in which data were collected (month/year to month/year).
Results
Present your results in logical sequence. Do not repeat in the text all the data in the tables or figures; emphasize or summarize only important observations. Subset Ns should accompany percentages if the total N is < 100. For original research, results should not be shown as not significant or NS. Actual P values are important for future meta-analyses research. Please include actual P values, and preferably confidence intervals or limits, when reporting nonsignificant results.
Discussion
Emphasize the new and important aspects of the study and the conclusions that follow from them. Do not repeat in detail material given in the Introduction or the Results section. Present in the Discussion section the implications of the findings and their limitations, including implications for future research. Relate the observations to other relevant studies. Link the conclusions with the goals of the study but avoid unqualified statements and conclusions not completely supported by your data.
Systematic Reviews and Meta-Analyses:
When preparing review articles and meta-analyses, describe the methods used in performing the literature review. This description includes listing the data sources searched (for example, MEDLINE) and the dates, keywords, and constraints (for example, language limits) used in the search; the criteria used to select the included studies; and the guidelines used for abstracting and synthesizing the data. Word limit for review articles and meta-analyses should not exceed 5,000 words of text. PRISMA guidelines should be followed, and a PRISMA Flowchart included as a figure. The journal considers a PRISMA Checklist submitted as supplementary material to be for reviewers’ information only and will not consider it intended for publication. The Checklist should be uploaded as a separate file labeled “PRISMA Checklist” with the “Supplementary material” file type selected.
In meta-analyses, several basic content areas should be addressed: study design, combinability, control of bias, statistical analysis, sensitivity analysis, and problems of applicability.
Please refer to the information on Abstracts for a more detailed framework of the necessary elements for review articles and meta-analyses.
Letters to the Editor (Includes Case Reports and Small Studies):
Letters must include a descriptive title, but they should not have an abstract. Most letters to the editor either report cases or small studies or comment on a recent journal article. Letters reporting small studies and case reports should not exceed 600 words and 1 table or figure. Letters commenting on a recent PCC article should not exceed 500 words.
Case reports should describe novel, well-documented findings that will be of use to the practitioner. Ensure that the chronology of events is clear; dates should be deidentified if used. A patient consent statement should be added to the title page of the manuscript indicating that consent was received from the patient to publish the case report and that information (including dates if applicable) has been deidentified to protect patient anonymity. Specify diagnostic criteria used for any diagnoses mentioned, and provide references for scales/assessment tools used. If a search of the literature was conducted for related case reports, specify the data sources, keywords, and any date/language limitations used in the search.
Letters reporting cases typically consist of (1) a brief introductory paragraph, (2) description of the cases, and (3) a discussion section. For letters reporting multiple cases, the types of clinical and demographic details given (eg, race, sex, occupation, marital status, medications, follow-up) should be consistent among the cases. It is not necessary to include all of these details in case reports, but if a particular characteristic, eg, occupation, is reported for one case, it should be reported for all of the cases.
Letters reporting small studies typically include (1) a brief introductory paragraph, (2) sections titled “Method” and “Results” (labeled with capitalized headings), and (3) a conclusions/discussion section. Ensure that the chronology of events is clear, and specify the month/year in which events occurred. Specify diagnostic criteria used for any diagnoses mentioned, and provide references for scales/assessment tools used. If a search of the literature was conducted, specify the data sources, keywords, and any date/language limitations used in the search.
For letters commenting on a Journal article, include a numbered reference to the article discussed. Be concise, and support your assertions with references as applicable. Also, please note that the authors of the original article will be given the opportunity to reply to letters commenting on their article.
Consensus Statements:
Consensus statements should identify the participants and their areas of expertise, as well as the source of funding or sponsor. Describe the data sources used and explain their selection, abstraction, and the method of their synthesis. A description of the process used to reach consensus should be included. Explain how conclusions were reached.
Please refer to the information on Abstracts for a more detailed framework of the necessary elements for consensus statements. Word limit for consensus statements should not exceed 5,000 words.
Bibliographic References:
The reference list should include only references to information that is retrievable. Authors are responsible for verifying the accuracy and completeness of the references against the original publications or presentations. Reference lists are limited to 75 references.
Citing References in Text and Graphics
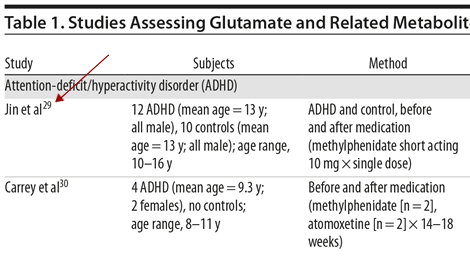
Reference List Style
The journal follows the AMA Manual of Style, 10th edition. Abbreviations of journal names must conform to Index Medicus style.
Examples
Unpublished Material:
Unpublished material includes personal communications and unpublished data such as data on file and manuscripts in preparation, submitted but not yet accepted, or in press.
Ways to include these citations and the requirements for each specific type of unpublished material:
Personal Communications
To include a personal communication from each person you identify as a source of information:
In the manuscript, authors must
In the submission system:
Unpublished Data
To include unpublished data from each person you identify as a source of information:
In the manuscript, authors must
In the submission system:
Unpublished Data in Meta-Analyses
When performing a meta-analysis, you may sometimes need to contact the author of an article you’re citing for information that was not published, eg, to learn the disposition of a subject or whether certain data were collected but not reported in the original study. If you contact these authors and further information is provided:
Data on File
Data on file from a pharmaceutical company are considered unpublished data.
In the manuscript, authors must
In the submission system:
Manuscripts Submitted, Forthcoming, or in Preparation
When citing an unpublished manuscript, consider that the status of the cited manuscript may change substantially from the time when your manuscript is submitted/accepted until the time it is published. Before citing unpublished data, consider whether doing so could jeopardize publication of the manuscript still in review, ie, whether publication of your manuscript could potentially “scoop” the data presented in the one still under review. If it could, then you should not publish outcomes reported in that manuscript, and the relevant information should be removed from your article.
Computer-generated figures should be submitted as separate files (minimum 300 dpi). Please provide . . .
Tables and figures should not duplicate text or one another and must be self-explanatory. Tables should be numbered consecutively in the order of their first citation in the text, as should figures. Acknowledge the original source of a previously published or adapted table or figure and submit written permission from the copyright holder to reproduce the material.
Footnotes. 3 types:
Tables. Identify each table by a brief descriptive title. Give each column a short heading. When percentages are presented, the appropriate numbers must also be given. Do not use internal horizontal and vertical rules. Place explanatory matter in footnotes, not in the headings or title. Units of measurement should be specified. Definitions of symbols appearing in tables should be listed at the end of the footnotes, with the expansions of abbreviations.
Figures. Two-dimensional graphs should not be represented in 3 dimensions. Figures are usually reduced to a width of 19.5 picas (3.25 in, 8.2 cm). Definitions of symbols appearing in the figure should be presented in a key (or legend) within the figure, rather than in the title or footnotes. The key should appear within or above the figure but should not widen the figure.
References Within Graphics. Ensure references embedded within a graphic are numbered consecutively with the text, from where graphic’s callout is first cited. See Citing References in Text and Graphics.
The journal accepts submissions of supplementary material. Supplementary material…
Appendixes: Appendixes will be published online only as supplementary material. Appendixes should be labeled as “Appendix 1,” “Appendix 2,” etc.
Tables and Figures: Label supplemental graphics—in text and on the graphics themselves—as “Supplementary Table 1,” “Supplementary Figure 1,” etc.
Text: For supplemental text, provide a heading describing the text. Use this heading in the manuscript to point the reader to the appropriate material, eg, “(see supplementary material for a list of excluded studies),” “(The MRI data acquisition and processing methodology can be found in supplementary material.),” “(A full list of search methods appears in supplementary material.)”
PRISMA and CONSORT Checklists and Flowcharts:
Checklists: For review articles and for randomized controlled trials, the journal considers PRISMA and CONSORT Checklists to be for reviewers’ information only and not intended for publication. The Checklist file should be uploaded separately in PDF format with the file type “Supplementary material” selected and labeled “PRISMA Checklist” or “CONSORT Checklist” as appropriate. However, if the author indicates the Checklist is submitted specifically for publication, the Checklist should then be uploaded in PDF format as supplementary material or included with other supplementary material in a combined PDF file.
Flowcharts: A PRISMA Flowchart must be supplied with review articles, and a CONSORT Flowchart must be supplied with randomized controlled trials. It is preferable that the flowchart be 1 of the 5 graphics to be published within the article.
Multiple Files: If multiple files are submitted, authors will be asked to append them into 1 PDF, with the exception of a Checklist not intended for publication, which should remain separate.
Back to top
The turnaround time from acceptance to publication is 3–5 months. Articles are edited on the basis of their accepted date and then published online soon after production is complete and author approval received.
The corresponding author will be notified when the article has been published online and will receive a complimentary PDF.
JCP does not charge submission or publication fees. However, charges are associated with some aspects of article processing. See the table of Article Processing Charges and the table of Article Access Options.
Contact the Publications Manager at [email protected].

