ABSTRACT
Objective: Young patients with intracerebral hemorrhage (ICH) make up a small but important subgroup of patients with ICH. This study investigated the clinical characteristics and outcomes of hypertensive ICH in very young (18–45 years) and young (46–55 years) patients.
Methods: This was a retrospective study of patients aged 18–55 years with hypertensive ICH admitted to a hospital from April 2014 to April 2019. Clinical and radiologic features as well as long-term clinical outcomes were compared between 2 age groups: group 1 (18–45 years) and group 2 (46–55 years). Factors affecting the clinical outcome were investigated as well.
Results: Of 63 patients with hypertensive ICH, 24 (38.1%) were in group 1 (mean ± SD age of 38 ± 4.6 years), and 39 (61.9%) were in group 2 (50 ± 2.5 years). The risk factor profile was similar except for diabetes, which was more prevalent in group 1 (odds ratio [OR] = 4.65; 95% CI, 1.4–15.2). Patients in group 1 had higher mean ± SD NIH Stroke Scale scores (15.7 ± 4.6, P = .044), had lower Glasgow Coma Scale (GCS) scores (OR = 3.33; 95% CI, 1.0–10.8), were at higher risk of intubation (OR = 2.79; 95% CI, 1.1–9.9), and had higher ICH volume (21 ± 18, P = .034). Worse clinical outcome was higher in group 1 (OR = 5.14; 95% CI, 1.0–26.1). Low GCS score, mean hematoma volume, and intraventricular extension were independently associated with worse outcome.
Conclusions: Relatively young patients with hypertensive ICH have higher prevalence of diabetes and worse clinical outcome in comparison to older patients with hypertensive ICH. Such patients should be monitored and treated more aggressively.
Prim Care Companion CNS Disord 2021;23(3):20m02768
To cite: Albakr A, AlFajri A, Almatar A, et al. Hypertensive intracerebral hemorrhage in young patients from a tertiary care center in Saudi Arabia: an observational study. Prim Care Companion CNS Disord. 2021;23(3):20m02768.
To share: https://doi.org/10.4088/PCC.20m02768
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Eastern Province, Saudi Arabia
bDepartment of Radiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Eastern Province, Saudi Arabia
cDepartment of Neurosurgery, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Eastern Province, Saudi Arabia
*Corresponding author: Noman Ishaque, FCPS, Department of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, King Faisal Rd, Dammam, 34212, Saudi Arabia ([email protected]).
Intracerebral hemorrhage (ICH) accounts for 3.7% to 38.5% of all cases of stroke in young patients with devastating consequences on socioeconomic aspects as well as quality of life.1 Hypertension has surpassed vascular malformation as the most common etiology of ICH in the young population.2–4 This change is secondary to increase in prevalence of vascular risk factors in younger individuals.5 Data regarding characteristics and outcome of ICH in young patients are very limited and mainly derived from studies conducted in middle-aged and elderly patients with ICH.6–15 However, data pertaining to middle-aged and elderly patients cannot be applied to younger patients because there are differences in clinical features; for example, younger patients with ICH are more likely to be smokers, to have excessive alcohol consumption, to be obese, and to have positive family history of ICH.3,16 Thus, it is extremely important that clinical features and outcomes of hypertensive ICH in young patients be explored to lead to focused management.
With the lack of data on hypertensive ICH in the younger age group in mind, we investigated various clinical and radiologic features of hypertensive ICH in patients aged 18 to 45 years and compared them with those of patients aged 46 to 55 years. We also compared outcomes of ICH between the 2 age groups and investigated any factors that could be associated with worse outcomes. We hypothesized that relatively younger patients with hypertensive ICH have different clinical characteristics as well as worse outcomes compared to older patients with hypertensive ICH.
METHODS
Study Population and Design
Records of patients aged 18 to 55 years who were admitted to King Fahd Hospital of University, Al Khobar, Saudi Arabia with ICH from April 2014 to April 2019 were reviewed. Of 230 patients, 63 were included in our study. Patients were excluded if they had subdural hematoma, epidural hematoma, subarachnoid hemorrhage, ICH caused by etiologies other than hypertension, or incomplete workup for ICH.
Subjects were divided into 2 groups: group 1 included patients aged 18 to 45 years and group 2 included patients aged 46 to 55 years. Patients’ clinical and radiologic characteristics as well as outcomes were compared between the 2 groups. The ethics review board of our institution approved the study.
Operational Definitions
ICH was defined as rapidly developing clinical signs of neurologic dysfunction attributable to focal collection of blood within brain parenchyma or ventricular system as seen on neuroimaging (head computed tomography [CT] or magnetic resonance imaging [MRI]) that is not caused by trauma.17 Hypertension was defined as systolic blood pressure > 140 mm Hg or diastolic blood pressure > 90 mm Hg that persisted for 14 days after presentation in a patient who was not known to have hypertension or in a patient who was known to have hypertension.18 Patients were designated as diabetic if they were using oral hypoglycemic agents or insulin at the time of presentation or had levels of fasting blood glucose > 126 mg/dL, postprandial blood glucose > 200 mg/dL, or hemoglobin A1c ≥ 6.5%.19 However, there is a limitation of this definition as oral hypoglycemic drugs are also prescribed for conditions other than diabetes or prediabetes. Impaired renal function was defined as glomerular filtration rate < 60 mL/min/1.73 m2. Creatinine clearance was calculated as an estimate of glomerular filtration rate according to the Chronic Kidney Disease Epidemiology Collaboration equation.20 Neuroimaging was evaluated by the neuroradiologist regarding location, hematoma volume, and intraventricular extension. Location of hematoma on neuroimaging was divided into either supratentorial or infratentorial. Supratentorial location was further subdivided into deep or nonlobar ICH if the hematoma was located in basal ganglia (internal or external capsule) or thalamus or lobar ICH if the hematoma was located in any 4 lobes of the cerebral hemispheres. Infratentorial location included hemorrhage into the brain stem or cerebellum. Hematoma location was divided into supratentorial and infratentorial because infratentorial hemorrhages are associated with increased odds of poor outcome, disability, and death.21 Lobar ICH is associated with better outcome and decreased odds of mortality but increased odds of recurrence in comparison to nonlobar ICH.22 ICH volume was measured via the “ABC/2 method” wherein A is the greatest hemorrhage diameter by CT, B is the diameter 90° to A, and C is the approximate number of CT slices with hemorrhage multiplied by the slice thickness.23 Hematoma expansion was defined as increase in hematoma volume by more than 6 mL or more than 33% from baseline volume on follow-up imaging at 24 hours.24–27
All patients underwent extensive radiologic and laboratory workup for ICH and hypertension as per institution protocol, which included CT or MR angiogram of the head; digital subtraction angiography; delayed brain imaging, preferably MRI 3 months after presentation; MRI of the head with and without contrast; transthoracic echocardiogram; 12-lead electrocardiography; blood workup for autoimmune vasculitis; and serum/urine toxicology screening depending on clinical suspicion.
Data Collection
Data collected included patient demographics; hypertension, diabetes, and ischemic heart disease status; low-density lipoprotein level; impaired renal function; current smoking status; alcohol use; and use of antiplatelet medications. All patients received standard of care in accordance with hospital protocol, which is based on guidelines for management of spontaneous ICH issued by the American Heart Association and American Stroke Association.28 All patients were treated using available antihypertensive medications with target systolic blood pressure < 140 mm Hg. Decisions regarding surgical intervention including external ventricular drain, craniotomy, and craniectomy were left to the treating physician and were individualized according to the patient’s clinical and radiologic characteristics. Mean systolic blood pressure was recorded at 24 hours. Patients who had a Glasgow Coma Scale (GCS)29 score ≤ 8 were intubated and put on mechanical ventilation. Decisions for neurosurgical procedures, if required for intraventricular hemorrhage, hydrocephalus, or in the case of neurologic deterioration, were left to the neurosurgical staff, and guidelines for management of spontaneous ICH issued by the American Heart Association and American Stroke Association28 were followed. Data for such neurosurgical interventions were collected.
Outcomes
The primary aim of this study was to compare the clinical and radiologic features as well as the long-term functional outcome between the 2 groups. Functional outcome was evaluated with the modified Rankin Scale (mRS)30 and was assessed by telephone interview of the patient or caregiver (minimum follow-up was at 6 months and maximum up to 1 year after ICH onset). A mRS score of 0–2 was considered favorable functional outcome, while 3–6 was considered unfavorable. The secondary aim of this study was to investigate factors that affect functional outcome in the 2 groups. Considering the results of previous ICH research, we selected the following factors for correlation with clinical outcome: diabetes, impaired renal function, GCS score, ICH location and volume, and intraventricular extension.
Data Analysis Procedures
Data were analyzed with IBM SPSS Statistics 21. All categorical variables were presented as frequencies and percentages. Quantitative variables (ie, age, systolic blood pressure, diastolic blood pressure, mean arterial pressure, NIH Stroke Scale [NIHSS],31 and hematoma volume) were presented as mean and standard deviation. Independent sample t test was used to compare those variables. χ2 test for association was used to compare the clinical features, risk factors, and radiologic features between age groups. For multivariate analysis, logistics regression was used to confirm the significant associated factors with age groups. Factors found to be significant in the univariate analysis were also analyzed in a logistic regression model to assess the independent predictors of functional outcome. Odds ratios (ORs) were calculated, and P value < .05 was considered significant.
RESULTS
Of 127 patients with ICH, the diagnosis was confirmed by noncontrast CT head scans in all patients (100%). Of those patients, 113 (88.97%) underwent further workup for secondary cause of ICH, while 14 (11.02%) had unclear etiology due to either incomplete investigations or early death. Of 113 patients with complete workup, 63 were found to have hypertensive ICH, while some underlying pathology was found in the remaining 50 patients. The analyses of baseline characteristics and outcome after ICH are restricted to those with hypertensive ICH. Of 63 patients with hypertensive ICH, 29 (46%) had prior history of hypertension, while the remaining 34 (53.92%) were diagnosed with hypertension-related ICH as they fulfilled the criteria mentioned previously.
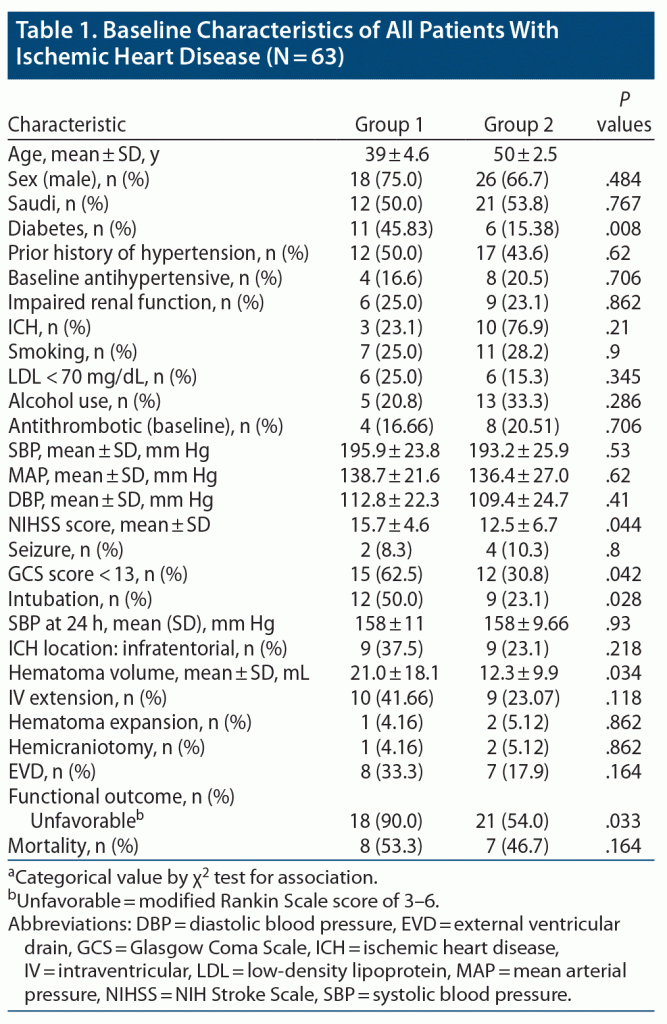
The majority of patients (n = 39, 61.9%) were in group 2 (mean ± SD age of 50 ± 2.5 years; range, 45–55 years), and the remaining patients (n = 24, 38.1%) were in group 1 (aged 39 ± 4.6 years; range, 30–44 years). When comparing group 1 with group 2 (Table 1), demographics were equally distributed in both age groups (P > .05). Risk factors, except for diabetes, did not differ significantly between the 2 groups. Diabetes was significantly associated with the younger age group (group 1: P = .008) (Table 1).
The mean NIHSS score at presentation was significantly higher in group 1 (15.7 ± 4.6, P = .044) compared to group 2 (12.5 ± 6.7). Reduced level of consciousness at admission with GCS score < 13 was significantly associated with patients in group 1 (n = 15, 62.5%, P = .042). Need for intubation was also significantly associated with patients in group 1 (n = 12, 50.0%, P = .028). Regarding location of ICH, supratentorial hematoma was more common than infratentorial, and location of ICH was equally distributed between both age groups. The mean hematoma volume was significantly higher in group 1 (21.0 ± 18.1) compared to group 2 (12.3 ± 9.9, P = .034). Noncontrast CT head scan was repeated within 24 hours of symptom onset in all patients, which showed hematoma expansion in 3 patients (4.76%), and its association with age groups was found to be insignificant. Association of mean systolic blood pressure reading at 24 hours after ICH onset showed statistically significant association with age groups (P = .93). External ventricular drain was inserted in 15 (23.8%) patients, and hemicraniectomy was done in 3 patients (4.76%). Association of surgical intervention and age groups was found to be insignificant.
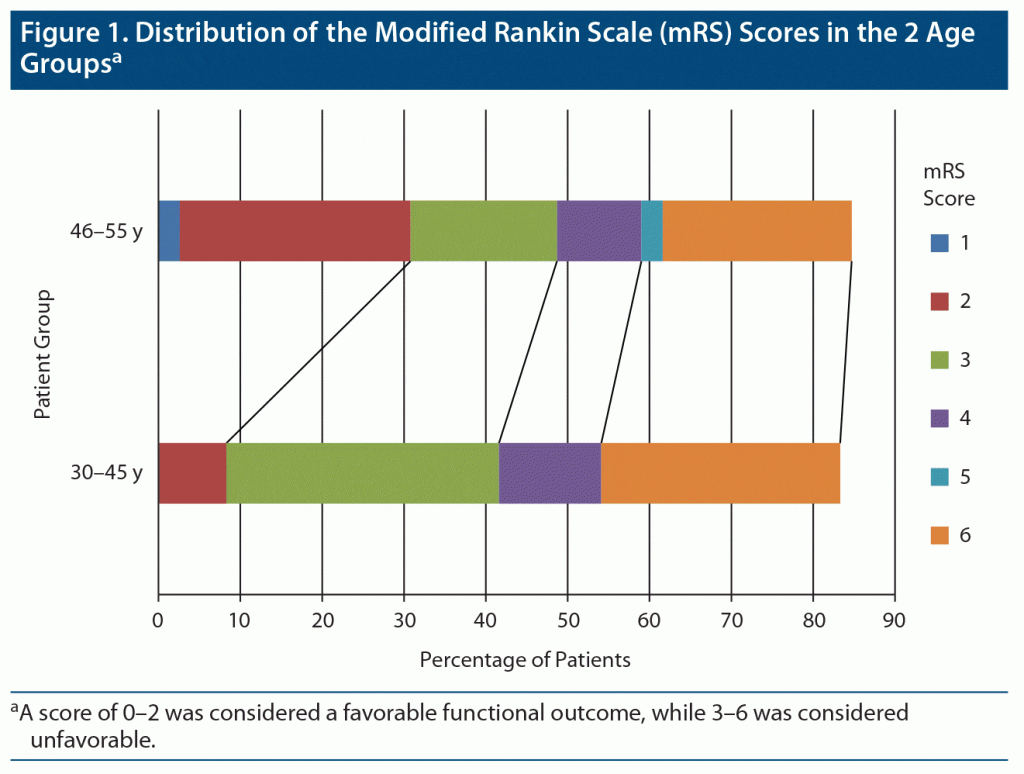
At the end of follow-up for 6–12 months, complete data on outcomes were available for 62 (98.4%) patients. All of the patients were functionally independent prior to onset of their symptoms. Overall mortality rate was 23.8%, and the frequency of death was equally distributed between both age groups (P = .164). By the end of the follow-up period, 39 patients had either died or were functionally dependent (mRS score of 3–6), and the proportion of patients with this unfavorable outcome was significantly associated with patients in group 1 (18, 90%, P = .033).The distribution of mRS scores between the 2 groups is presented in Figure 1.
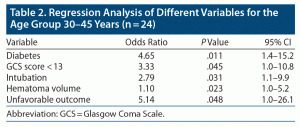
Logistic regression analysis of all significant variables between age groups is presented in Table 2. Unfavorable outcome was 5.14 times higher in younger patients compared to older patients (aged 46–55 years, P = .048). Diabetes was 4.65 times more prevalent in group 1 compared to group 2 (P = .011).
A GCS score < 13 was 3.33 times more prevalent in younger patients (P = .045), and need for intubation was 2.79 times higher in group 1 (P = .031).
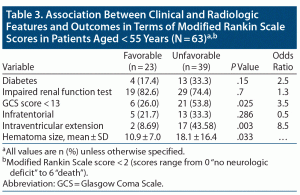
Factors associated with favorable or unfavorable functional outcome for all patients are shown in Table 3. Intraventricular extension was 8.5 times more prevalent in patients with worse clinical outcome (P = .003). Need for mechanical ventilation was 3.86 times higher (P = .028) and GCS score < 13 was 3.5 times higher (P = .025) in patients with unfavorable outcomes. The mean hematoma volume (18.1 ± 16.4, P = .033) was significantly high in patients with unfavorable outcomes.
DISCUSSION
From this retrospective analysis, we observed that (1) there was no significant difference in prevalence of risk factors based on age at onset except for diabetes, which was more prevalent in younger patients; (2) there were increased odds of having low GCS scores at time of admission and need for intubation in younger patients; (3) younger patients had higher NIHSS scores, hematoma volume, and odds of having unfavorable functional outcome; and (4) low GCS score, large hematoma volume, and intraventricular extension were independent factors for poor clinical outcome in younger patients with ICH.
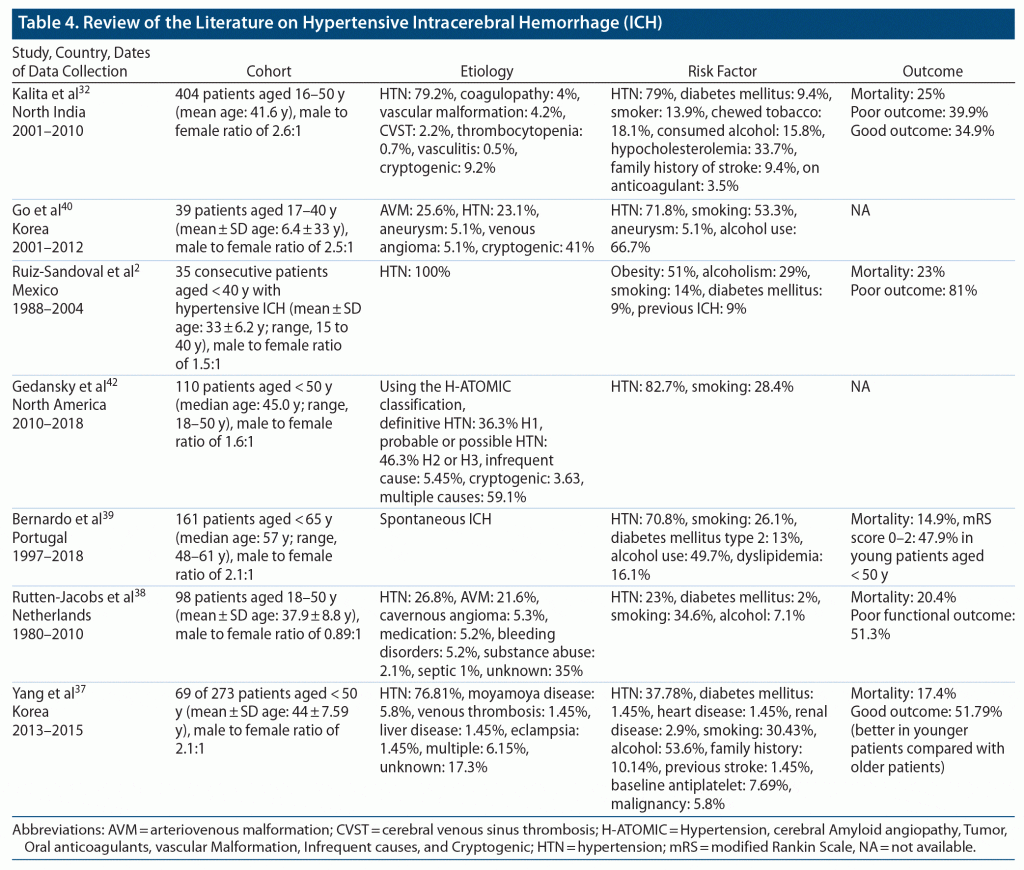
There is no uniform definition of ICH in young patients, as age criteria varies in studies from 18 to 45 years as shown in Table 4. Hypertension is the most common cause of ICH in younger patients, so we wanted to investigate whether there is any difference in clinical or radiologic features and outcome in relatively younger patients with hypertensive ICH in comparison to older patients.
Hypertension has been recognized as the most common cause of ICH in the younger population in previous studies (Table 4). Only 50.0% of patients in group 1 and 43.6% of patients in group 2 were known to have hypertension at the time of presentation with ICH. So, close to half of all the patients were not diagnosed with hypertension prior to presentation with ICH. This finding highlights the importance of early screening for hypertension, as it is the most modifiable risk factor for ICH. Moreover, our study showed that only 41% of patients with preexisting hypertension were taking antihypertensives at the time of the ICH event. This finding might indicate that hypertension is often underestimated in young individuals and stresses the role of public education and awareness as well.
All vascular risk factors except diabetes were equally distributed in both age groups and were relatively similar to that reported in a previous study by Kalita et al.32 Although the reported prevalence of diabetes in Saudi Arabia based on the age groups 30–39, 40–49, and 50–59 years is 12.1%, 31.9%, and 58.2%, respectively,33,34 surprisingly, diabetes was significantly higher in group 1 compared with group 2. Although there is inconsistency among studies in establishing diabetes as a risk factor for ICH, few studies have reported increased prevalence of diabetes in younger patients with ICH or increased risk of ICH in Black younger patients with ICH.35 Currently, there is no plausible explanation for increased prevalence of diabetes in younger patients with ICH, but we think it is related to the changes in the cultural background/society norms of our subjects. More precisely, Saudis have experienced rapid economic development, including rapid changes in dietary habits, and reduction in physical activities during the past decades. As a result, Saudis are prone to increased insulin resistance, increased metabolic syndrome, and a higher visceral fat level within a similar body mass index range as Westerners.36 This finding should be investigated further.
Regarding the clinical and radiologic features, as somewhat expected, most of the presenting features, except for NIHSS scores, altered sensorium, and need for intubation, were similar in both groups and were consistent with results from previous studies.37–40 As a result of the multivariate analysis, patients in group 1 were found to be 3 times more likely to have low GCS scores and a 2 times higher need for intubation. Our results might be partially explained by a significant higher rate of hematoma volume (P = .034) and slightly higher rate of brain stem location in group 1 compared with group 2 (29.7% vs 12.8%), although this rate was not clinically significant, which could be attributed to ICH severity in the younger group as well. We also noticed that 31% of patients had intraventricular extension, and 28.6% of patients underwent surgical intervention. The frequency of surgical intervention was similar to that previously reported by Ruiz-Sandoval et al2 and Koivunen et al.41 As expected, considering the radiologic and clinical features in group 1, surgical interventions were performed more in this group.
Previous studies38,41,42 have shown that relatively younger patients in a cohort of young patients with ICH have better functional outcome compared to relatively older patients who have poor functional outcome. Our study revealed unexpected results, as worse clinical outcome was 5.14 times more common in patients in group 1. One explanation for this finding is that all the factors that were found to be significantly contributing to worse outcome were more prevalent in group 1 compared to group 2. It is important for clinicians to recognize the potential factors for worse clinical outcome in young patients with ICH to optimize outcomes. As expected, severe ICH presentation with low GCS score, large hematoma volume, and intraventricular extension were significantly associated with long-term poor clinical outcome, and that was similar to results reported by Koivunen et al.41
Our study has some limitations. (1) The study was a single-center, retrospective design, and there may have been underreporting of cases or incomplete data, mainly regarding the clinical symptoms, risk factors, and mRS scores. (2) Functional outcome was measured with the mRS administered by telephone interview. Scores might have been underestimated or overestimated, particularly in cases in which the mRS score was reported by a caregiver. (3) Our study included patients aged 18–55 years, while most previous studies only included patients less than < 50 years of age. (4) The small sample, with none of the included patients younger than age 30, could limit generalization. We assume that our findings of clinical outcome might be underpowered by the small sample size. (5) Matching on multiple variables would be difficult due to the small pool of young patients with ICH and thus confounding could arise. (6) Considering that cerebral amyloid angiopathy (CAA) is a very rare cause of ICH in younger individuals, there have been few case reports of CAA as an underlying etiology of ICH in this age group.43,44 Modified Boston Criteria using head MRI should also be considered in young patients, mainly in lobar ICH.45
Despite our limitations, we think this study highlights that hypertension-related ICH in younger individuals is a real burden with high fatality and poor clinical outcome. Importantly, ICH in younger individuals is thought to more likely be the result of a complex interplay of many risk factors, and additional contribution of a single or few factors may have a bigger role in its development. Therefore, screening programs at the community level and clinical strategies to control modifiable ICH risk factors might help to reduce the burden of this disabling disease.
CONCLUSION
Relatively young patients with hypertensive ICH have higher prevalence of diabetes and worse clinical outcome in comparison to older patients with hypertensive ICH. In primary care settings, hypertension and diabetes were important modifiable risk factors for all subtypes of strokes. Considering that hypertension is an independent risk factor for morbidity and mortality in patients with ICH, this comorbidity should be managed aggressively in young patients as part of a primary prevention strategy related to ICH. Further large prospective studies with detailed clinical and radiologic assessment of ICH events in the young population and their outcome are therefore required.
Submitted: July 27, 2020; accepted November 11, 2020.
Published online: May 27, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Clinical Points
- Relatively young patients with hypertensive intracerebral hemorrhage (ICH) have higher prevalence of diabetes and worse clinical outcomes compared to older patients.
- Predictors of unfavorable functional outcome in patients with ICH include age at presentation, low Glasgow Coma Scale score, large hematoma volume, and intraventricular extension.
- Hypertension in young patients should be identified and treated to reduce the burden of ICH.
References (45)

- Marini C, Russo T, Felzani G. Incidence of stroke in young adults: a review. Stroke Res Treat. 2010;2011:535672. PubMed
- Ruiz-Sandoval JL, Romero-Vargas S, Chiquete E, et al. Hypertensive intracerebral hemorrhage in young people: previously unnoticed age-related clinical differences. Stroke. 2006;37(12):2946–2950. PubMed CrossRef
- Tatlisumak T, Cucchiara B, Kuroda S, et al. Nontraumatic intracerebral haemorrhage in young adults. Nat Rev Neurol. 2018;14(4):237–250. PubMed CrossRef
- Broderick M, Rosignoli L, Lunagariya A, et al. Hypertension is a leading cause of nontraumatic intracerebral hemorrhage in young adults. J Stroke Cerebrovasc Dis. 2020;29(5):104719. PubMed CrossRef
- George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 2017;74(6):695–703. PubMed CrossRef
- Jacobs BS, Boden-Albala B, Lin IF, et al. Stroke in the young in the northern Manhattan stroke study. Stroke. 2002;33(12):2789–2793. PubMed CrossRef
- Marini C, Totaro R, De Santis F, et al. Stroke in young adults in the community-based L’Aquila registry: incidence and prognosis. Stroke. 2001;32(1):52–56. PubMed CrossRef
- Bevan H, Sharma K, Bradley W. Stroke in young adults. Stroke. 1990;21(3):382–386. PubMed CrossRef
- Leno C, Berciano J, Combarros O, et al. A prospective study of stroke in young adults in Cantabria, Spain. Stroke. 1993;24(6):792–795. PubMed CrossRef
- Lin CL, Howng SL. Nontraumatic intracerebral hemorrhage in young adult. Kaohsiung J Med Sci. 1997;13(4):237–242. PubMed
- Ruíz-Sandoval JL, Cantú C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes, and prognosis. Stroke. 1999;30(3):537–541. PubMed CrossRef
- Del Brutto OH, Sánchez J, Campos X, et al. Non-traumatic intracerebral hemorrhage in young adults living in Guayaquil, Ecuador (South America): analysis of 151 patients. Funct Neurol. 1999;14(1):21–28. PubMed
- Lai SL, Chen ST, Lee TH, et al. Spontaneous intracerebral hemorrhage in young adults. Eur J Neurol. 2005;12(4):310–316. PubMed CrossRef
- Ruiz-Sandoval JL, Ortega-Alvarez L, García-Navarro V, et al. Intracerebral haemorrhage in a referral hospital in the central-western region of Mexico [in Spanish]. Rev Neurol. 2005;40(11):656–660. PubMed
- Barinagarrementeria F, Ruiz-Sandoval JL, Cantu C. Hypertensive intracerebral hemorrhage in young people. Neurology. 2001;56(suppl 3):A433.
- van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. PubMed CrossRef
- Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. PubMed CrossRef
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. PubMed CrossRef
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80. CrossRef
- Lesley A, Marcie A, Christopher H, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. PubMed
- Delcourt C, Sato S, Zhang S, et al; INTERACT2 Investigators. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology. 2017;88(15):1408–1414. PubMed CrossRef
- Samarasekera N, Fonville A, Lerpiniere C, et al; Lothian Audit of the Treatment of Cerebral Haemorrhage Collaborators. Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke. 2015;46(2):361–368. PubMed CrossRef
- Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305. PubMed CrossRef
- Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1–5. PubMed CrossRef
- Thompson AL, Kosior JC, Gladstone DJ, et al; PREDICTS/Sunnybrook ICH CTA Study Group. Defining the CT angiography ‘spot sign’ in primary intracerebral hemorrhage. Can J Neurol Sci. 2009;36(4):456–461. PubMed CrossRef
- Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40(9):2994–3000. PubMed CrossRef
- Chang EF, Meeker M, Holland MC. Acute traumatic intraparenchymal hemorrhage: risk factors for progression in the early post-injury period. Neurosurgery. 2006;58(4):647–656, discussion 647–656. PubMed CrossRef
- Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. PubMed CrossRef
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. PubMed CrossRef
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. PubMed CrossRef
- Lyden P, Brott T, Tilley B, et al; NINDS TPA Stroke Study Group. Improved reliability of the NIH Stroke Scale using video training. Stroke. 1994;25(11):2220–2226. PubMed CrossRef
- Kalita J, Goyal G, Kumar P, et al. Intracerebral hemorrhage in young patients from a tertiary neurology center in North India. J Neurol Sci. 2014;336(1-2):42–47. PubMed CrossRef
- Al Dawish MA, Robert AA, Braham R, et al. Diabetes mellitus in Saudi Arabia: a review of the recent literature. Curr Diabetes Rev. 2016;12(4):359–368. PubMed CrossRef
- Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011;31(1):19–23. PubMed CrossRef
- Hesami O, Kasmaei HD, Matini F, et al. Relationship between intracerebral hemorrhage and diabetes mellitus: a case-control study. J Clin Diagn Res. 2015;9(4):OC08–OC10. PubMed
- Alzeidan RA, Rabiee F, Mandil AA, et al. Changes in dietary habits, physical activity and status of metabolic syndrome among expatriates in Saudi Arabia. East Mediterr Health J. 2018;23(12):836–844. PubMed CrossRef
- Yang NR, Kim JH, Ahn JH, et al. Is nontraumatic intracerebral hemorrhage different between young and elderly patients? Neurosurg Rev. 2020;43(2):781–791. PubMed CrossRef
- Rutten-Jacobs LC, Maaijwee NA, Arntz RM, et al. Clinical characteristics and outcome of intracerebral hemorrhage in young adults. J Neurol. 2014;261(11):2143–2149. PubMed CrossRef
- Bernardo F, Rebordão L, Machado S, et al. In-hospital and long-term prognosis after spontaneous intracerebral hemorrhage among young adults aged 18-65 years. J Stroke Cerebrovasc Dis. 2019;28(11):104350. PubMed CrossRef
- Go GO, Park H, Lee CH, et al. The outcomes of spontaneous intracerebral hemorrhage in young adults—a clinical study. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):214–220. PubMed CrossRef
- Koivunen RJ, Tatlisumak T, Satopää J, et al. Intracerebral hemorrhage at young age: long-term prognosis. Eur J Neurol. 2015;22(7):1029–1037. PubMed CrossRef
- Gedansky A, Jarvis P, Yu D, et al. Intracerebral hemorrhage in a young urban population: etiologies and outcomes in patients 50 and younger. J Stroke Cerebrovasc Dis. 2019;28(10):104295. PubMed CrossRef
- Purrucker JC, Hund E, Ringleb PA, et al. Cerebral amyloid angiopathy—an underdiagnosed entity in younger adults with lobar intracerebral hemorrhage? Amyloid. 2013;20(1):45–47. PubMed CrossRef
- Malik MT, Myers C, Kazmi SJ, et al. Recurrent intracerebral hemorrhage after exercise in a young patient presenting with sporadic cerebral amyloid angiopathy in a young patient. J Vasc Interv Neurol. 2017;9(6):12–13. PubMed
- Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston Criteria. Stroke. 2018;49(2):491–497. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite