ABSTRACT
Importance: While eating disorders (EDs) affect people of all ages, reproductive stages, and genders, they are most prevalent in women of reproductive age and can have a profound impact on fertility and obstetric outcomes. Due to the high prevalence and health consequences, EDs in this group of women require specific attention.
Objective: To discuss the implications of EDs in infertility, pregnancy, and the postpartum period and to introduce tools to aid in identifying disordered eating and appropriate treatment recommendations for women with suspected EDs.
Evidence Review: A comprehensive literature search was conducted of articles available on PubMed, last updated retrieval date March 27, 2023. Chain searching was used to identify other relevant articles. The following search terms were included: (pregnancy OR postpartum) AND (bulimia nervosa OR eating disorder OR anorexia nervosa OR binge eating disorder) AND (obstetric outcome OR infant outcome OR infant development OR depression OR anxiety); (fertility OR infertility) AND (bulimia nervosa OR eating disorder OR anorexia nervosa OR binge eating disorder OR weight suppression OR eating disorder not otherwise specified OR other specified feeding and eating disorder OR atypical anorexia nervosa OR binge eating OR low weight); and eating disorders AND PCOS. Articles pertinent to the impact of eating disorders on fertility and the impact of perinatal eating disorders on infant and mother were selected.
Findings: Perinatal EDs impact maternal mental health and obstetric and infant outcomes. They can have a long-lasting effect on the offspring via epigenetic changes. EDs are also a common and treatable cause of infertility.
Conclusions and Relevance: Recognition and treatment of EDs in women prior to conception can minimize obstetric risks to the woman and potential long-term adverse effects on the offspring. For women with infertility, recognition and treatment of EDs can increase the probability of conception.
Prim Care Companion CNS Disord 2023;25(4):22nr03475
Author affiliations are listed at the end of this article.
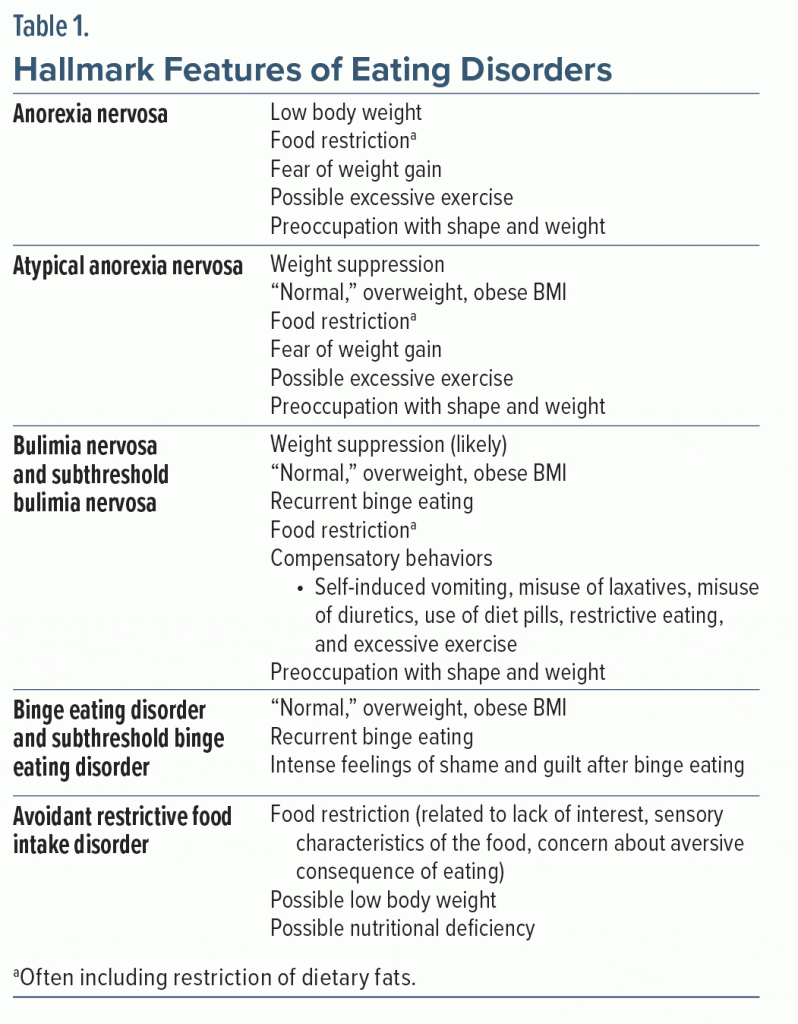
Eating disorders (EDs) are highly prevalent in women of reproductive age, with the majority of EDs beginning by age 25 years.1 Consequently, EDs often start prior to pregnancy or attempting to conceive. A disturbance in food intake is common to all EDs; however, each ED has unique features (Table 1). Anorexia nervosa (AN) is characterized by dietary restriction, and it results in significantly low weight, an intense fear of gaining weight, an over evaluation of shape and weight, and often a lack of concern regarding the seriousness of malnutrition. The primary features of bulimia nervosa (BN) are recurrent episodes of binge eating and compensatory behaviors to prevent weight gain, along with an over evaluation of shape and weight. Binge eating describes eating a large amount of food often very quickly and experiencing a loss of control. Compensatory behaviors to prevent weight gain can include self-induced vomiting, misuse of laxative, misuse of diuretics, ingestion of diet pills, restrictive eating, and/or excessive exercise. Binge eating disorder (BED) is identified by recurrent episodes of binge eating, which are often associated with intense feelings of distress, guilt, and shame. Other specified feeding and eating disorders (OSFED) include atypical anorexia nervosa, subthreshold BN and BED, purging disorder, and night eating syndrome.2 Atypical anorexia nervosa is characterized by a significant weight loss that does not result in an objectively low body mass index (BMI). Purging disorder is marked by recurrent vomiting to influence shape and weight without binge eating. Night eating syndrome is identified by overnight awakenings to eat after falling asleep. Avoidant restrictive food intake disorder (ARFID) is a persistent failure to meet adequate dietary intake, often related to a lack of interest in eating, a fear of an aversive consequence of eating (eg, pain, choking), or a concern about the sensory characteristics of the food.2
METHODS
A comprehensive literature search was conducted of articles available on PubMed, last updated retrieval date March 27, 2023. The following search terms were included: (pregnancy OR postpartum) AND (bulimia nervosa OR eating disorder OR anorexia nervosa OR binge eating disorder) AND (obstetric outcome OR infant outcome OR infant development OR depression OR anxiety); (fertility OR infertility) AND (bulimia nervosa OR eating disorder OR anorexia nervosa OR binge eating disorder OR weight suppression OR eating disorder not otherwise specified OR other specified feeding and eating disorder OR atypical anorexia nervosa OR binge eating OR low weight); and eating disorders AND PCOS.
The authors reviewed titles and abstracts to select articles available in English that were pertinent to the impact of eating disorders on fertility and the impact of perinatal eating disorders on infant and mother. Once relevant articles were identified, citation chaining for additional articles was conducted. Selected articles were reviewed to inform the corresponding sections.
RESULTS
Prevalence and Perinatal Course of Eating Disorders
The combined lifetime prevalence for the most common EDs, including AN, BN, and BED, is around 6%.3 This is likely a conservative estimate, as EDs are frequently underrecognized, even in mental health settings4 and especially among racial and ethnic minority populations.5 OSFED, sometimes referred to as subthreshold EDs, are frequently missed.6 The prevalence rates for newer ED diagnoses, such as ARFID, are not yet fully known.7
EDs are common among women seeking fertility treatment, with rates ranging from 0.5% to 16.7%.8–10 Lifetime ED prevalence is particularly high (95.2%) in women requiring pulsatile gonadotropin-releasing hormone (pGnRH) therapy.11 EDs, especially BED and OSFED, are also common in the perinatal period. Roughly 5%–7.5% of pregnant women meet criteria for an ED.12,13 The relapse rates for EDs in pregnancy and the postpartum period range from 25% to 67%.14–16 Some women experience a decrease in ED behaviors, primarily food restriction and self-induced vomiting, during pregnancy.16 However, these behaviors usually return and even increase in severity postpartum.17 The temporary improvement during pregnancy may be related to a change in perception of eating as necessary to “feed the baby” and to improvements in body image.18 However, it should be noted that ED behaviors, particularly binge eating, can intensify during pregnancy in some women.12
Implications of Active Eating Disorders
Effect on fertility. The impact of EDs on reproductive health and fertility is well documented.10,19 The reproductive consequences of EDs are primarily mediated through changes in weight and the subsequent impact on hormone levels and menses. Certain ED behaviors are known to suppress the hypothalamic-pituitary-ovarian (HPO) axis. These ED behaviors include restriction of overall caloric intake, restriction of macronutrient intake such as dietary fat, and excessive exercise. Suppression of the HPO axis causes functional hypothalamic amenorrhea (FHA) and anovulation due to reduction in pGnRH.20 Of note, food restriction does not always result in an objectively low BMI, but rather a weight that is too low for one’s body. This “weight suppression” is often not visible; nevertheless, food restriction leading to weight suppression can cause serious consequences similar to being medically underweight,21 including suppression of the HPO axis.22
ED behaviors such as binge eating or a long history of dieting can result in overweight and obesity. Overweight and obesity contribute to hormonal imbalances that lead to anovulation, menstrual irregularities, longer duration to pregnancy, reduced response to fertility treatment, and higher rates of infertility.23 While weight loss is often a treatment recommendation to overweight and obese patients seeking fertility treatment in general, such a recommendation for individuals with a current or past ED may not be achievable and is associated with increased risk of ED relapse.24 In cases wherein weight loss is indicated to improve fertility, this recommendation should be offered only after carefully screening for an ED. If a past or current ED is identified, weight loss recommendations may not be appropriate and a referral for ED treatment should be considered.
Women with BN and BED may be at higher risk of developing polycystic ovarian syndrome (PCOS),25 which compromises fertility. While overweight, obesity, and PCOS are highly comorbid, the relationship of these disorders and EDs is not fully understood.26
Effect on perinatal women. EDs alter maternal weight, and both high and low maternal BMI increase obstetric risk. Women with EDs have differences in preconception, pregnancy, and postpartum weights, as well as perinatal weight trajectories, compared to women without EDs.27 Preconception and gestational weights, in turn, affect obstetric outcomes. High maternal BMI increases a woman’s risk for gestational diabetes, hypertensive disorders of pregnancy, and cesarean section.28 Low maternal BMI, on the other hand, has been associated with anemia and preterm delivery.28,29 Consequently, it is imperative to detect EDs in reproductive-aged women to diminish the obstetric risks of high and low maternal weight.
Women with EDs are also at increased risk of specific obstetric complications. Antenatal active ED symptoms in general are associated with risk of cesarean section.30 In addition, maternal AN increases a woman’s risk of preterm delivery.31 Importantly, obstetric risks can be mitigated by treating the disorder prior to conception. For example, women with active BN are at higher risk of miscarriage and preterm delivery compared to women with treated BN.32
In addition to the physical complications, antenatal EDs have been negatively associated with maternal mood. Women with active antenatal ED symptoms are at higher risk for both perinatal anxiety and depression,30 which contribute to maternal morbidity and mortality.33–38
While treating EDs prior to pregnancy is imperative for women’s health, women with EDs in remission continue to require close monitoring in the perinatal period. The perinatal period is a high-risk time for relapse of ED, with one-quarter of women in remission prior to pregnancy relapsing during this time.14
Breastfeeding may also be negatively impacted by the presence of an ED, although the literature is inconclusive.39,40 A recent systematic review41 highlights 2 important findings when comparing women with EDs to women without EDs. First, women with EDs, especially those with AN, are likely to breastfeed for less time. Second, women with EDs experience more difficulty (eg, worries about milk insufficiency and pain) breastfeeding. Conceptualizing breastfeeding as a possible maladaptive weight control and purging behavior requires consideration.42 We have anecdotally observed some women with EDs in our practice excessively breastfeeding or pumping to influence their shape and weight. This area remains largely understudied, with the exception of a small (N = 16) qualitative study43 on breastfeeding practices in women with EDs that found that the primary motivations to breastfeed included losing weight, returning to pre-pregnancy body shape, and permission to eat higher calorie avoided foods without the fear of weight gain.
Effect on offspring. Perinatal maternal weight and nutritional status influence fetal development. Preconception weight and gestational weight influence neonatal outcomes. High maternal BMI is a risk factor for delivering an infant who is large for gestational age.28 Low maternal BMI, in contrast, has been associated with preterm birth and intrauterine growth retardation.28,29 Maternal EDs have also been directly linked to specific adverse infant outcomes. Active antenatal ED symptoms in general are associated with the neonate having lower 1-minute Apgar scores and being large or small for gestational age.30 Maternal AN, specifically, is associated with preterm birth prior to 37 weeks, still birth, and having a neonate born at low birth weight, small for gestational age, with neonatal jaundice, with cardiovascular disorders, with respiratory disorders, and who is at higher risk of admission to the neonatal intensive care unit.31 Women with EDs also engage at higher rates in behaviors that can adversely affect fetal as well as obstetric health including smoking.44
The effect of in utero and neonatal exposure to maternal EDs extends beyond early life and can have a long-lasting impact on offspring. Maternal EDs can affect offspring into adulthood through genetics, fetal programming, and learned behaviors.
In utero exposure to a maternal ED can alter fetal programming, such as through epigenetic changes.45 Epigenetic changes involve those in DNA methylation and histone modification that influence gene expression across life.
Inadequate caloric and nutritional intake antenatally, regardless of presence of maternal ED, have been associated with fetal epigenetic changes that affect the offspring’s health, including risk of developing metabolic syndrome later in life.46,47 Epigenetic changes are thought to mediate the associations between in utero exposure to folic acid deficiency and both autism spectrum disorder (ASD) and neural tube defects.48,49
Learned behaviors also contribute to the effect of maternal EDs on offspring. Mothers with EDs are more likely to have concerns about their infant becoming overweight, to have trouble identifying whether the infant is hungry or full, to report the infant is having feeding problems, and to have infants with different growth trajectories after adjusting for birth weight.50–54 Furthermore, these early experiences have a long-lasting effect and increase the child’s future risk of developing EDs.55,56
Screening for Eating Disorders
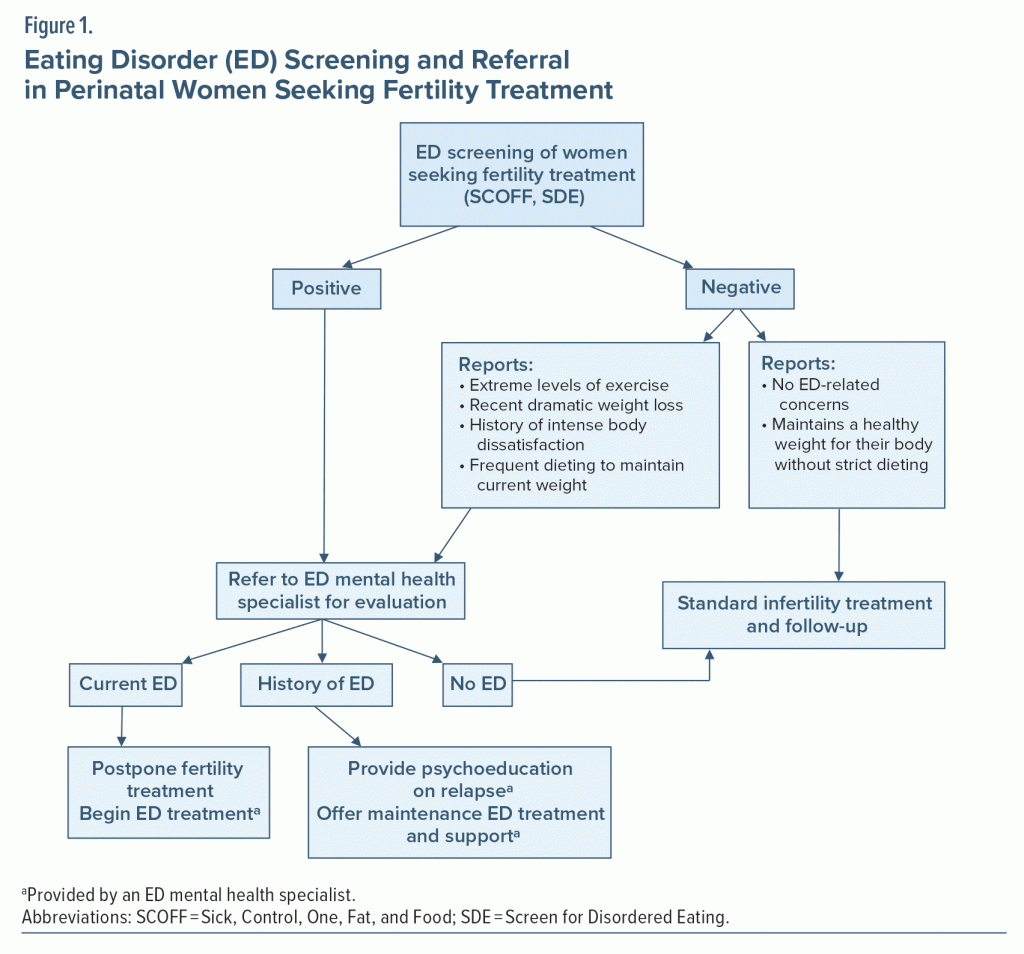
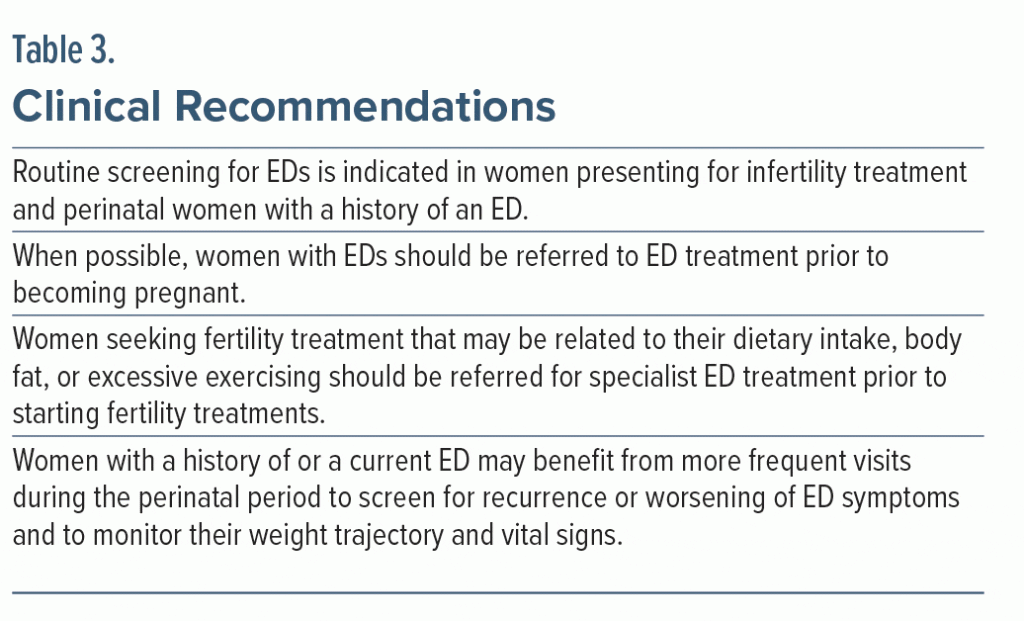
Given the prevalence of EDs and impact on mothers and their offspring, it is important to screen women of reproductive age for EDs wherever they access care. Useful times include yearly physicals with their primary care providers, family planning checkups, obstetric appointments, or appointments with fertility specialists. We especially recommend screening women as follows: when they initially present with infertility, if fertility treatment response has been less than expected, at the first antenatal appointment, and at their postpartum appointment. Women who screen positive should then be referred to mental health providers trained to diagnose, treat, and monitor symptoms through the perinatal period.
Specific ED screening tools for use during pregnancy are limited, and no postpartum-specific tools exist. A commonly used, very brief self-administered screening tool in nonpregnant women is the Sick, Control, One, Fat, and Food (SCOFF). The SCOFF contains 5 questions and has satisfactory sensitivity for detecting AN and BN57; however, it is less accurate for detecting BED and OSFED.58 The 5-item self-administered Screen for Disordered Eating more accurately identifies symptoms of BED (again in nonpregnant samples).59 Recently, the Prenatal Eating Behaviors Screening (PEBS) Tool60 was developed to screen for EDs in pregnancy. The 12-item measure performed well during initial psychometric testing using self-reported ED diagnosis, but further research is needed, especially in clinical settings. In addition to screening measures, given the impact of excessive exercise and weight suppression on fertility, we suggest including questions about both during the clinical patient interview.
Despite the high prevalence of EDs in women presenting for fertility treatment and the repercussions of EDs on fertility treatment, ED training for fertility specialists is limited, and many report feeling ill equipped to identify EDs in their patients.61 Screening for EDs in women seeking fertility treatment (Figure 1) is particularly important because identification of an ED and referral for treatment may reduce the need for infertility-related interventions and increase the success of infertility treatment. Special attention is needed in carefully screening women in fertility clinics who may find disclosing such information difficult.19 Self-administered screens combined with an interview style that is warm and expresses knowledge about ED behaviors is likely to encourage accurate reporting from patients. Finally, screening male partners for EDs when male factor infertility is identified may also be warranted due to the impact of weight and excessive exercise on male fertility.62
Treatments
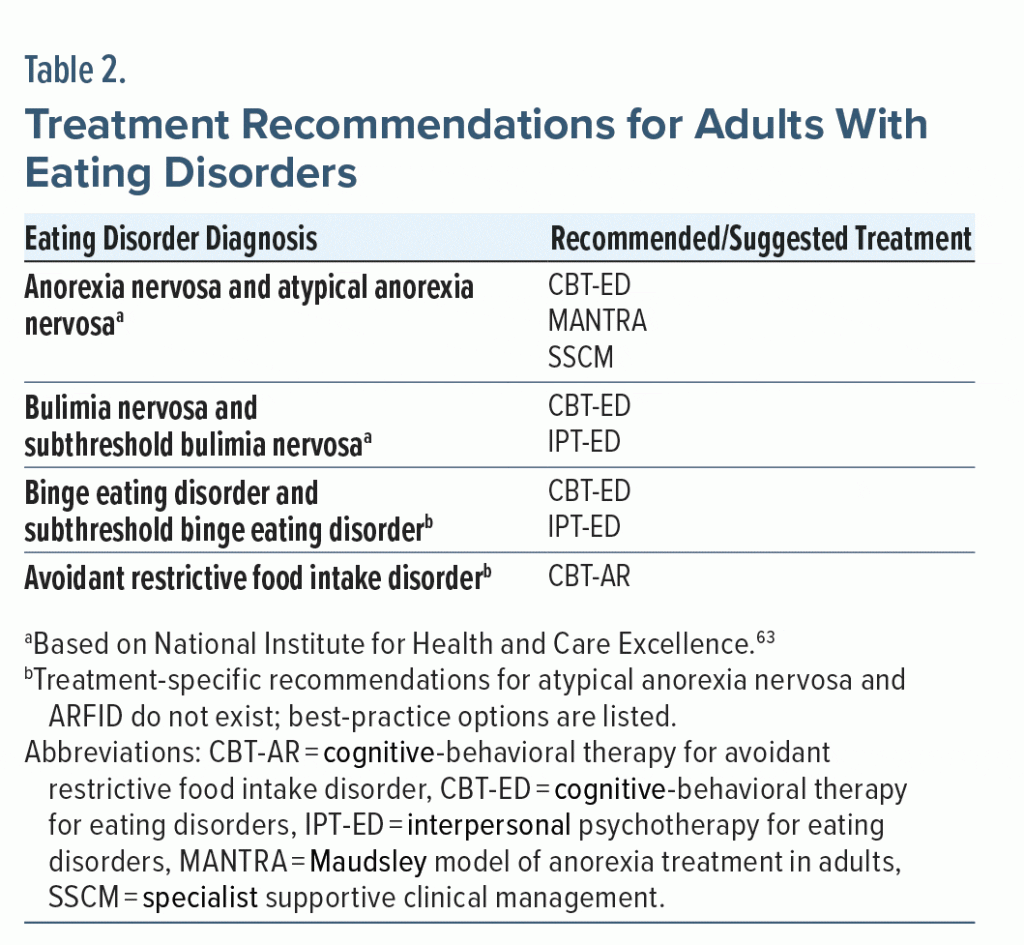
EDs are treatable and respond favorably to well-delivered protocols. Recommended treatments for EDs primarily consist of outpatient behavioral therapies, including time-limited cognitive-behavioral therapy (CBT-ED) and interpersonal psychotherapy (IPT-ED).63 ED psychotherapy recommendations for adults who do not require weight gain and adults who do require weight gain differ (Table 2). Most patients can be treated with outpatient therapy; however, higher levels of care would be recommended if outpatient treatment fails.
For some, the severity of ED behavior is too severe to manage on an outpatient basis. Day treatment programs, residential hospital programs, psychiatric hospitalization, or medical hospitalization may be required. The presence of recent and precipitous weight loss (ie, ≥ 4 lb over a 3-week period), critical laboratory values (eg, hypokalemia, hyponatremia, hypophosphatemia, and metabolic acidosis or alkalosis), ECG abnormalities (eg, prolonged QTc, bradycardia, other arrhythmias), and hemodynamic instability are all potential indicators requiring medical stabilization. Specific guidelines indicating when pregnant patients with EDs require higher levels of psychiatric or medical treatment do not exist.64 We recommend utilizing the guidelines for nonpregnant patients; however, the threshold for hospitalization may be lower given the potential harmful consequences of ongoing ED behavior during pregnancy for the woman and fetus.
Few pharmacologic interventions have proved effective for EDs. There are currently 2 US Food and Drug Administration–approved medications for EDs. Fluoxetine is approved for the treatment of BN, and lisdexamfetamine is approved for the treatment of BED. Fluoxetine is well studied in pregnancy and may be an appropriate addition to psychotherapy in the perinatal period in certain women who do not adequately respond to psychotherapy alone.65 Prior to use of fluoxetine in the perinatal period, it is important to have a discussion with a woman about the potential benefits and risks of fluoxetine for that particular woman, as well as the potential risks of that woman’s undertreated ED. Lisdexamfetamine improves abstinence from binging, lowers weight, and improves triglyceride levels.66 However, lisdexamfetamine is poorly studied in pregnancy.
If infertility appears related to ED behavior, referring to ED-specific psychological treatment should be considered the first step. Treating the ED may prevent unnecessary fertility treatments (ie, injectable pulsatile GnHR67). Indeed, in low weight patients with EDs, assisted reproductive technology is contraindicated.68 Reducing over exercising and improving nutrition and weight are the treatment of choice for these women. It is important to note that hormone replacement therapy and oral contraceptive pills (OCPs) do not treat amenorrhea. OCPs induce withdrawal bleeding; however, this can be misinterpreted as an indicator of improved health and fertility.69
Pregnant women or women in preconception who screen positive for an ED should be referred to an ED behavioral health specialist. When possible, women with EDs should receive ED-specific treatment prior to becoming pregnant. Identification and treatment prior to pregnancy is important in 2 ways. First, treatment before pregnancy mitigates the perinatal risks associated with an ED. Second, the ED can be more successfully treated with evidence-based psychotherapy outside of pregnancy due to both a lack of data and inability to implement the standard ED treatment protocols in pregnancy. Unfortunately, no specific treatment recommendations are available for women who are currently pregnant and experiencing an ED. Pregnancy has frequently been one of the few exclusion criteria for eating disorder treatment trials. The rationale has been that targeting one’s eating habits, views of their body, and/or interpersonal relationships all while the body is undergoing tremendous changes and weight gain are incompatible. Although active full protocol psychological treatment (eg, CBT-ED) will likely be delayed until the postpartum period, certain components of ED treatment can be applied in pregnancy. The main components that can be implemented in pregnancy include ED-support, ensuring adequate nutrition and hydration; guidance on appropriate eating patterns; and techniques for reducing certain ED behaviors (ie, self-induced vomiting; laxative, diuretic, and diet pill misuse; and overexercising). Women who need assistance with adequate weight gain during pregnancy or who are struggling to accept pregnancy-related weight gain likely will benefit from education regarding the nutritional requirements of the fetus and guidance on expected pregnancy weight changes. This component should involve a registered dietitian for assessment and management, as well as frequent collaboration with the obstetrics team.
For women in the postpartum period, in addition to individual psychological therapy, treatment that includes video or live in-session feedback of mother-baby interactions during feeding sessions may be particularly helpful. These sessions allow the therapist (and later the patient if a recording is used) to observe and identify any feeding challenges (ie, the mother not making eye contact with the baby during feeding) and to guide the mother to change these behaviors. Reducing mealtime conflict and supporting appropriate meal interaction improve mother-baby interactions generally and reduce ED psychopathology in mothers.70
While the ideal treatment plan involves evidence-based psychological therapy by an ED specialist, this is unfortunately not always possible to obtain. In these cases, we recommend that patients be referred to a general mental health provider and have more frequent contact with their obstetrics team and/or primary care provider. During the medical appointments, weight, vital signs, ED symptoms, and laboratory work can be monitored.
SUMMARY
EDs negatively impact perinatal women and their offspring. Identifying and monitoring women of reproductive age for EDs can profoundly influence health outcomes and reduce the need for certain fertility treatments. When possible, diagnosing and treating EDs prior to conception is recommended. By recognizing and referring these women for ED treatment, the medical and psychiatric consequences of EDs on a woman and her offspring can be minimized. Women with a history of an ED are at high risk of relapse during the perinatal period and require close monitoring (Table 3). Despite evidence in the literature of the consequences of active perinatal EDs, investigation of perinatal-specific ED symptoms and treatment for perinatal women with EDs is scarce. More research focused on EDs throughout the perinatal period is needed. Areas for future focus include the development of screening tools that include the postpartum period, studies on the prevalence of women with EDs using breastfeeding and pumping as an ED-related behavior, and the development of evidence-based ED treatments for pregnant and postpartum women.
Article Information
Published Online: August 31, 2023. https://doi.org/10.4088/PCC.22nr03475
© 2023 Physicians Postgraduate Press, Inc.
Submitted: December 21, 2022; accepted April 4, 2023.
To Cite: Bailey-Straebler SM, Susser LC. The impact of eating disorders on reproductive health: mitigating the risk. Prim Care Companion CNS Disord. 2023;25(4):22nr03475.
Author Affiliations: Weill Cornell Medicine—New York Presbyterian Hospital, White Plains (Bailey-Straebler and Susser); Columbia University Medical Center, New York (Bailey-Straebler).
Author Contributions: Drs Bailey-Straebler and Susser are both first co-authors.
Corresponding Author: Suzanne Bailey-Straebler, PhD, MSN, 21 Bloomingdale Road, White Plains NY 10605 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Acknowledgments: The authors are grateful to Samantha Berlow, LMSW (New York Presbyterian/Weill Cornell Medicine) for her help in preparing this manuscript. Samantha Berlow has no conflicts of interest related to the subject of this article.
Previous Presentation: Symposium presented on a similar topic at the Academy of Consultation-Liaison Psychiatry Annual Meeting, Consultation-Liaison Psychiatry; Atlanta, Georgia; November 2022.
ORCID: Suzanne Bailey-Straebler: https://orcid.org/0000-0002-2846-1330
Clinical Points
- Screening for eating disorders in women presenting for fertility treatment can help detect reversible causes of infertility and improve fertility treatment success.
- The perinatal period is a high-risk time for eating disorder relapse, and women may benefit from closer psychiatric monitoring.
- Women with eating disorder symptoms in pregnancy should be screened for perinatal anxiety and depression due to elevated risk.
- Treating an eating disorder prior to conception can mitigate adverse outcomes for women and their offspring.
References (70)

- Udo T, Grilo CM. Prevalence and correlates of DSM-5–defined eating disorders in a nationally representative sample of US adults. Biol Psychiatry. 2018;84(5):345–354. PubMed CrossRef
- American Psychiatric Association. American Psychiatric Association: DSM-5. Washington, DC: American Psychiatric Association; 2013.
- Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358. PubMed CrossRef
- Fursland A, Watson HJ. Eating disorders: a hidden phenomenon in outpatient mental health? Int J Eat Disord. 2014;47(4):422–425. PubMed CrossRef
- Goel NJ, Jennings Mathis K, Egbert AH, et al. Accountability in promoting representation of historically marginalized racial and ethnic populations in the eating disorders field: a call to action. Int J Eat Disord. 2022;55(4):463–469. PubMed CrossRef
- Harrop EN, Mensinger JL, Moore M, et al. Restrictive eating disorders in higher weight persons: A systematic review of atypical anorexia nervosa prevalence and consecutive admission literature. Int J Eat Disord. 2021;54(8):1328–1357. PubMed CrossRef
- Harshman SG, Jo J, Kuhnle M, et al. A moving target: how we define avoidant/restrictive food intake disorder can double its prevalence. J Clin Psychiatry. 2021;82(5):20m13831. PubMed CrossRef
- Freizinger M, Franko DL, Dacey M, et al. The prevalence of eating disorders in infertile women. Fertil Steril. 2010;93(1):72–78. PubMed CrossRef
- Le Floch M, Crohin A, Duverger P, et al. Prevalence and phenotype of eating disorders in assisted reproduction: a systematic review. Reprod Health. 2022;19(1):38. PubMed CrossRef
- Hecht LM, Hadwiger A, Patel S, et al. Disordered eating and eating disorders among women seeking fertility treatment: a systematic review. Arch Women Ment Health. 2022;25(1):21–32. PubMed CrossRef
- Barbosa-Magalhaes I, Corcos M, Galey J, et al. Prevalence of lifetime eating disorders in infertile women seeking pregnancy with pulsatile gonadotropin-releasing hormone therapy. Eat Weight Disord. 2021;26(2):709–715. PubMed CrossRef
- Easter A, Bye A, Taborelli E, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21(4):340–344. PubMed CrossRef
- Pettersson CB, Zandian M, Clinton D. Eating disorder symptoms pre- and postpartum. Arch Women Ment Health. 2016;19(4):675–680. PubMed CrossRef
- Sollid C, Clausen L, Maimburg RD. The first 20 weeks of pregnancy is a high-risk period for eating disorder relapse. Int J Eat Disord. 2021;54(12):2132–2142. PubMed CrossRef
- Makino M, Yasushi M, Tsutsui S. The risk of eating disorder relapse during pregnancy and after delivery and postpartum depression among women recovered from eating disorders. BMC Pregnancy Childbirth. 2020;20(1):323. PubMed CrossRef
- Dörsam AF, Preißl H, Micali N, et al. The impact of maternal eating disorders on dietary intake and eating patterns during pregnancy: a systematic review. Nutrients. 2019;11(4):840. PubMed CrossRef
- Crow SJ, Agras WS, Crosby R, et al. Eating disorder symptoms in pregnancy: a prospective study. Int J Eat Disord. 2008;41(3):277–279. PubMed CrossRef
- Fogarty S, Elmir R, Hay P, et al. The experience of women with an eating disorder in the perinatal period: a meta-ethnographic study. BMC Pregnancy Childbirth. 2018;18(1):121. PubMed CrossRef
- Paslakis G, de Zwaan M. Clinical management of females seeking fertility treatment and of pregnant females with eating disorders. Eur Eat Disord Rev. 2019;27(3):215–223. PubMed CrossRef
- Jahraus J. Medical complications of eating disorders. Psychiatr Ann. 2018;48(10):463–467. CrossRef
- Peebles R, Sieke EH. Medical complications of eating disorders in youth. Child Adolesc Psychiatr Clin N Am. 2019;28(4):593–615. PubMed CrossRef
- Kerns J, Itriyeva K, Fisher M. Etiology and management of amenorrhea in adolescent and young adult women. Curr Probl Pediatr Adolesc Health Care. 2022;52(5):101184. PubMed CrossRef
- Best D, Bhattacharya S. Obesity and fertility. Horm Mol Biol Clin Investig. 2015;24(1):5–10. PubMed
- Grilo CM, Pagano ME, Stout RL, et al. Stressful life events predict eating disorder relapse following remission: six-year prospective outcomes. Int J Eat Disord. 2012;45(2):185–192. PubMed CrossRef
- Paganini C, Peterson G, Stavropoulos V, et al. The overlap between binge eating behaviors and polycystic ovarian syndrome: an etiological integrative model. Curr Pharm Des. 2018;24(9):999–1006. PubMed CrossRef
- Pirotta S, Barillaro M, Brennan L, et al. Disordered eating behaviours and eating disorders in women in australia with and without polycystic ovary syndrome: a cross-sectional study. J Clin Med. 2019;8(10):1682. PubMed CrossRef
- Zerwas SC, Von Holle A, Perrin EM, et al. Gestational and postpartum weight change patterns in mothers with eating disorders. Eur Eat Disord Rev. 2014;22(6):397–404. PubMed CrossRef
- Verma A, Shrimali L. Maternal body mass index and pregnancy outcome. J Clin Diagn Res. 2012;6(9):1531–1533. PubMed CrossRef
- Kosa JL, Guendelman S, Pearl M, et al. The association between pre-pregnancy BMI and preterm delivery in a diverse southern California population of working women. Matern Child Health J. 2011;15(6):772–781. PubMed CrossRef
- Chan CY, Lee AM, Koh YW, et al. Course, risk factors, and adverse outcomes of disordered eating in pregnancy. Int J Eat Disord. 2019;52(6):652–658. PubMed CrossRef
- Ante Z, Luu TM, Healy-Profitós J, et al. Pregnancy outcomes in women with anorexia nervosa. Int J Eat Disord. 2020;53(5):403–412. PubMed CrossRef
- Morgan JF, Lacey JH, Chung E. Risk of postnatal depression, miscarriage, and preterm birth in bulimia nervosa: retrospective controlled study. Psychosom Med. 2006;68(3):487–492. PubMed CrossRef
- Grigoriadis S, Wilton AS, Kurdyak PA, et al. Perinatal suicide in Ontario, Canada: a 15-year population-based study. CMAJ. 2017;189(34):E1085–E1092. PubMed CrossRef
- Mochache K, Mathai M, Gachuno O, et al. Depression during pregnancy and preterm delivery: a prospective cohort study among women attending antenatal clinic at Pumwani Maternity Hospital. Ann Gen Psychiatry. 2018;17(1):31. PubMed CrossRef
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. PubMed CrossRef
- Doktorchik C, Premji S, Slater D, et al. Patterns of change in anxiety and depression during pregnancy predict preterm birth. J Affect Disord. 2018;227:71–78. PubMed CrossRef
- Weissman MM, Wickramaratne P, Nomura Y, et al. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163(6):1001–1008. PubMed CrossRef
- Borchers LR, Dennis EL, King LS, et al. Prenatal and postnatal depressive symptoms, infant white matter, and toddler behavioral problems. J Affect Disord. 2021;282:465–471. PubMed CrossRef
- Allen KL, Gibson LY, McLean NJ, et al. Maternal and family factors and child eating pathology: risk and protective relationships. J Eat Disord. 2014;2(1):11. PubMed CrossRef
- Torgersen L, Ystrom E, Siega-Riz AM, et al. Maternal eating disorder and infant diet. A latent class analysis based on the Norwegian Mother and Child Cohort Study (MoBa). Appetite. 2015;84:291–298. PubMed CrossRef
- Kaß A, Dörsam AF, Weiß M, et al. The impact of maternal eating disorders on breastfeeding practices: a systematic review. Arch Women Ment Health. 2021;24(5):693–708. PubMed CrossRef
- Bailey-Straebler SM, Susser LC, Cooper Z. Breastfeeding and pumping as maladaptive weight control behaviors [published online ahead of print June 1, 2023]. Int J Eat Disord. 2023:eat.24006. PubMed CrossRef
- Stapleton H, Fielder A, Kirkham M. Breast or bottle? eating disordered childbearing women and infant-feeding decisions. Matern Child Nutr. 2008;4(2):106–120. PubMed CrossRef
- Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child cohort study (MoBa). Int J Eat Disord. 2009;42(1):9–18. PubMed CrossRef
- Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab. 2008;22(1):1–16. PubMed CrossRef
- Yan S, Hou W, Wu H, et al. Prenatal exposure to the Chinese famine and the risk of metabolic syndrome in adulthood across consecutive generations. Eur J Clin Nutr. 2020;74(8):1229–1236. PubMed CrossRef
- Dunford AR, Sangster JM. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: a systematic review. Diabetes Metab Syndr. 2017;11(suppl 2):S655–S662. PubMed CrossRef
- Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75(2):176–184. PubMed CrossRef
- Wald NJ, Law MR, Morris JK, et al. Quantifying the effect of folic acid. Lancet. 2001;358(9298):2069–2073. PubMed CrossRef
- Cimino S, Cerniglia L, Porreca A, et al. Mothers and fathers with binge eating disorder and their 18–36 months old children: a longitudinal study on parent-infant interactions and offspring’s emotional-behavioral profiles. Front Psychol. 2016;7:580. PubMed CrossRef
- Martini MG, Taborelli E, Schmidt U, et al. Infant feeding behaviours and attitudes to feeding amongst mothers with eating disorders: a longitudinal study. Eur Eat Disord Rev. 2019;27(2):137–146. PubMed CrossRef
- Micali N, Simonoff E, Treasure J. Infant feeding and weight in the first year of life in babies of women with eating disorders. J Pediatr. 2009;154(1):55–60.e1. PubMed CrossRef
- Micali N, Simonoff E, Stahl D, et al. Maternal eating disorders and infant feeding difficulties: maternal and child mediators in a longitudinal general population study. J Child Psychol Psychiatry. 2011;52(7):800–807. PubMed CrossRef
- Perrin EM, Von Holle A, Zerwas S, et al. Weight-for-length trajectories in the first year of life in children of mothers with eating disorders in a large Norwegian cohort. Int J Eat Disord. 2015;48(4):406–414. PubMed CrossRef
- Stein A, Woolley H, Cooper S, et al. Eating habits and attitudes among 10-year-old children of mothers with eating disorders: longitudinal study. Br J Psychiatry. 2006;189(4):324–329. PubMed CrossRef
- Nicholls DE, Viner RM. Childhood risk factors for lifetime anorexia nervosa by age 30 years in a national birth cohort. J Am Acad Child Adolesc Psychiatry. 2009;48(8):791–799. PubMed CrossRef
- Hill LS, Reid F, Morgan JF, et al. SCOFF, the development of an eating disorder screening questionnaire. Int J Eat Disord. 2010;43(4):344–351. PubMed
- Kutz AM, Marsh AG, Gunderson CG, et al. Eating disorder screening: a systematic review and meta-analysis of diagnostic test characteristics of the SCOFF. J Gen Intern Med. 2020;35(3):885–893. PubMed CrossRef
- Maguen S, Hebenstreit C, Li Y, et al. Screen for disordered eating: improving the accuracy of eating disorder screening in primary care. Gen Hosp Psychiatry. 2018;50:20–25. PubMed CrossRef
- Claydon EA, Lilly CL, Ceglar JX, et al. Development and validation across trimester of the Prenatal Eating Behaviors Screening tool. Arch Women Ment Health. 2022;25(4):705–716. PubMed CrossRef
- Rodino IS, Byrne SM, Sanders KA. Eating disorders in the context of preconception care: fertility specialists’ knowledge, attitudes, and clinical practices. Fertil Steril. 2017;107(2):494–501. PubMed CrossRef
- Deyhoul N, Mohamaddoost T, Hosseini M. Infertility-related risk factors: a systematic review. IJWHR. 2017;5(1):24–29. CrossRef
- Eating disorders: recognition and treatment: Guidance. NICE website. Accessed November 2, 2022. https://www.nice.org.uk/guidance/ng69
- Raykos B, Watson H. Treating Eating Disorders in Pregnancy and the Postpartum Period. In: Tortolani CC, ed. Adapting Evidence-Based Eating Disorder Treatments for Novel Populations and Settings. 1st ed. Routledge; 2020:238-267.
- Walsh BT, Agras WS, Devlin MJ, et al. Fluoxetine for bulimia nervosa following poor response to psychotherapy. Am J Psychiatry. 2000;157(8):1332–1334. PubMed CrossRef
- Brownley KA, Berkman ND, Peat CM, et al. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med. 2016;165(6):409–420. PubMed CrossRef
- Gibson MES, Fleming N, Zuijdwijk C, et al. Where have the periods gone? The evaluation and management of functional hypothalamic amenorrhea. J Clin Res Pediatr Endocrinol. 2020;12(suppl 1):18–27. PubMed CrossRef
- Academy of Eating Disorders. Eating Disorders: A Guide to Medical Care. 4th ed. 2021. AED website. Accessed November 20, 2022. https://higherlogicdownload.s3.amazonaws.com/AEDWEB/27a3b69a-8aae-45b2-a04c-2a078d02145d/UploadedImages/Publications_Slider/2120_AED_Medical_Care_4th_Ed_FINAL.pdf
- McIver B, Romanski SA, Nippoldt TB. Evaluation and management of amenorrhea. Mayo Clin Proc. 1997;72(12):1161–1169. PubMed CrossRef
- Gibson D, Workman C, Mehler PS. Medical complications of anorexia nervosa and bulimia nervosa. Psychiatr Clin North Am. 2019;42(2):263–274. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite