ABSTRACT
Objective: To synthesize the neurobiological basis of brain-resetting effects of psilocybin and identify neuroimaging correlates of psilocybin response in depressed patients.
Data Sources: MEDLINE(R), Embase, APA PsycINFO, Cochrane, and CINAHL were systematically searched on June 3, 2022, with no date restrictions using the following string: (psilocybin) AND (psychedelics) AND (MRI) OR (fMRI)) OR (PET)) OR (SPECT)) OR (imaging)) OR (neuroimaging)).
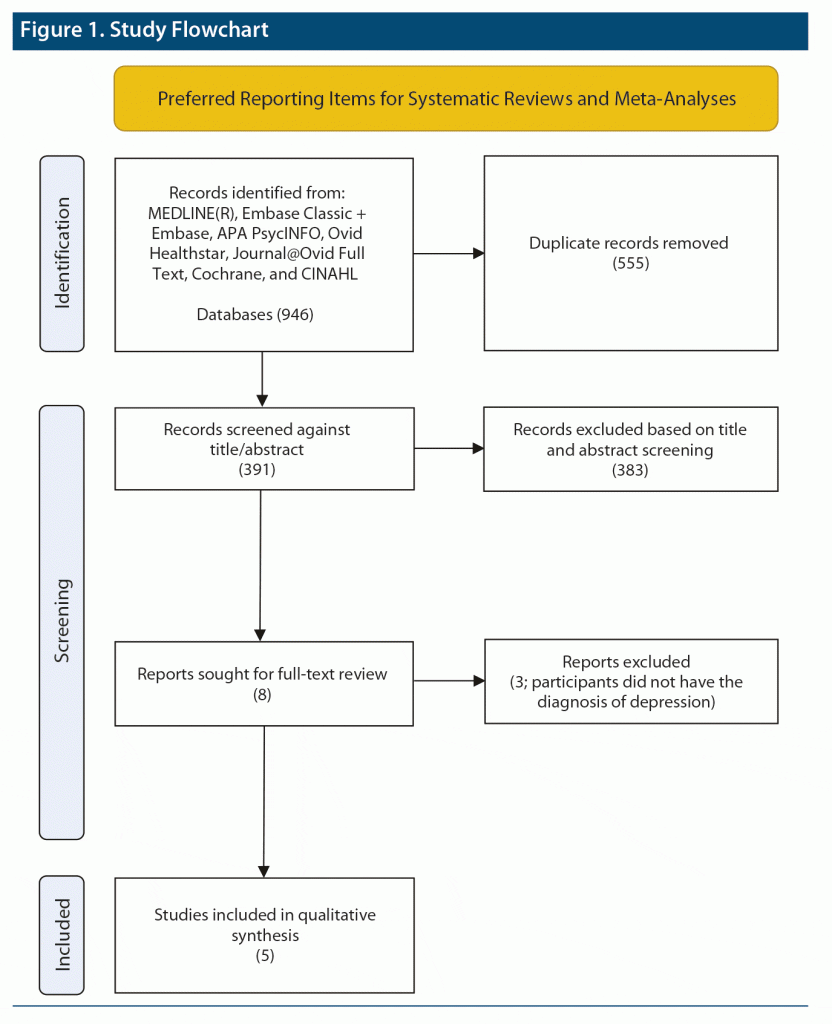
Study Selection: After duplicates were removed from 946 studies, 391 studies remained, of which 8 qualified for full-text analysis, but only 5 fulfilled the eligibility criteria of randomized, double-blind, or open-label neuroimaging study with psilocybin treatment in depressed patients.
Data Extraction: The Covidence platform was used for deduplication and bias assessment. The a priori data points included concomitant psychological intervention, modality of neuroimaging technique, changes in depression scores, brain functional changes, and association between functional and psilocybin response. Assessment bias was assessed with the standard risk of bias tool for randomized controlled trials and the tool for risk of bias in nonrandomized studies of interventions.
Results: Four studies were open-label, and one was a combined open-label and randomized controlled trial using functional magnetic resonance imaging. Psilocybin-assisted psychotherapy was administered in 3 studies, 1 in refractory and 2 in nonrefractory patients. The remaining 2 studies were in refractory patients. The transient increase in psilocybin-induced global connectivity in major neural tracts and specific areas of brain activation was associated with antidepressant response.
Conclusions: Transient functional brain changes with psilocybin therapy resemble the “brain reset” phenomenon and may serve as the putative predictors of psilocybin antidepressant response.
Prim Care Companion CNS Disord 2023;25(3):22r03419
To cite: Qasim S, Zaheer Z, Jawad MY, et al. Neurobiological correlates of psilocybin response in depression. Prim Care Companion CNS Disord. 2023;25(3):22r03419.
To share: https://doi.org/10.4088/PCC.22r03419
© 2023 Physicians Postgraduate Press, Inc.
aKing Edward Medical University, Lahore, Pakistan
bCentre for Addiction and Mental Health, Toronto, Ontario, Canada
cUniversity of Nevada Las Vegas, Nevada.
dTouro University Nevada College of Osteopathic Medicine, Las Vegas, Nevada
eThe Valley Health System, Las Vegas, Nevada
*Corresponding author: Saleha Qasim, MBBS, 1400 Bayou Shore Dr, Apt 207, Galveston, TX 77551 ([email protected]).
Despite significant pharmacologic advances, two-thirds of patients with major depressive disorder (MDD) do not respond to their antidepressant treatment. Finding the most effective treatment is often time-consuming due to the relatively early onset of adverse effects and delayed response and leads to medication nonadherence, poor prognosis, high socioeconomic burden, and loss of productive years.1,2 An increasing number of patients with failed interventions and a longer duration of unremitted depression are among the main reasons for an increased number of patients with treatment-refractory depression.3 Despite well-recognized improvement in neurovegetative symptoms, the most frequently used class of antidepressants, selective serotonin reuptake inhibitors (SSRIs), have been associated with various adverse effects, including cognitive haze and emotional blunting.4 Therefore, it becomes imperative to develop novel antidepressants that are rapidly effective across treatment-refractory and treatment-naive populations to achieve premorbid functionality and subjective improvements such as quality of life. The US Food and Drug Administration approval of intranasal esketamine, often labeled as a psychedelic, has reignited interest in exploring the antidepressant effects of other psychedelics, such as psilocybin and 3,4-methylenedioxymethamphetamine. Although esketamine is rapidly effective and has antisuicidal effects, it is expensive and requires 2-hour posttreatment monitoring due to the elevated risk for adverse effects.5 In addition, patient registration in the risk evaluation and mitigation services is needed before esketamine can be prescribed in a supervised setting.6
Moreover, ketamine-induced activation of μ-opioid receptors adds a potential risk for addiction.7,8 After a long hiatus, the resurrection of psychedelic research has produced promising results in managing several other psychiatric disorders, including obsessive-compulsive disorder, posttraumatic stress disorder, substance use disorder, and existential depression in terminal medical illnesses.9–11 In a head-to-head comparison, 57% of the patients receiving psilocybin-assisted therapy remitted with a faster onset of efficacy than 28% of those receiving escitalopram, an SSRI, with similar tolerability.12 More recently, depressed subjects produced a 75% response and 58% remission for at least 12 months after being randomized to receive immediate or delayed psilocybin-assisted psychotherapy.13 Psilocybin-assisted psychotherapy recently produced a significantly better response and a faster onset of efficacy than escitalopram.14
Of note, no adverse effects were reported at the psilocybin doses used in these studies. However, in a recently published large-sample psilocybin trial, the most frequent adverse events reported in the 25-mg group included headache, nausea, dizziness, and fatigue.15 However, few participants also developed serious adverse events of suicidal ideation and non-suicidal self-injurious behavior in the 25-mg and 10-mg dose groups. Of note, several participants already had suicidal thoughts and self-injurious behavior at baseline.15 Regardless, the findings from this study warrant clinical monitoring for suicidality in future trials of psilocybin in depressed patients. In addition, the study did not report any psychosis or altered perceptions in the study subjects.15 Nevertheless, higher psilocybin doses have been reported to induce psychosis and visual hallucinations, including the “bad trips,” which is not unexpected as psilocybin induces significant changes in perception including visual hallucinations, synesthesia, and altered emotions.16 Bad trips may involve confusion, irritability, anxiety, fear, psychosis, and frightening visions.17 In a survey, people using psilocybin reported risk of dose-related physical harm, particularly those who used psilocybin without supportive therapy and social support, which underscores the safer and more effective profile of psilocybin-assisted therapy.18 However, despite reported adverse effects most survey respondents reported psilocybin use as a therapeutic experience.19 Some studies20,21 have reported no adverse outcomes with psilocybin.
However, in general, rapid-onset and sustained antidepressant effects of psilocybin have increased interest in conducting neuroimaging studies to understand the neurobiological basis of the unique action profile of psilocybin. Although most neuroimaging studies have been conducted in healthy volunteers, there are growing data to demystify neurobiological mechanisms underlying psilocybin response. This review provides a synopsis of neuroimaging findings with psilocybin in depressed patients, comparing results from healthy volunteers and earlier neuroimaging findings with monoaminergic antidepressants.
METHODS
This review was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).22 Figure 1 provides the study flowchart.
Search Strategy
The online databases MEDLINE(R), Embase Classic + Embase, APA PsycINFO, Ovid Healthstar, Journal@Ovid Full Text, Cochrane, and CINAHL were systematically searched on June 3, 2022, with no date restrictions using the following string: (psilocybin) AND (psychedelics) AND (MRI) OR (fMRI)) OR (PET)) OR (SPECT)) OR (imaging)) OR (neuroimaging)). Only English-language studies were retrieved.
Eligibility Criteria
The review was primarily aimed at synthesizing the pretreatment and posttreatment changes in different functional areas and neural circuits of the brain after patients with MDD were administered a therapeutic dosage of psychedelics, primarily focusing on psilocybin and secondarily to find putative neuroimaging biomarkers that can predict or inform foregoing treatment. Hence, the following criteria were formed for the selection of studies following PRISMA guidelines23:
Population. Adult patients aged ≥ 18 years with a diagnosis of MDD according to the DSM–IV or DSM-5.
Intervention. A therapeutic dosage of a psychedelic is administered with an intent to treat (ITT) analysis.
Comparison group(s). Antidepressant, placebo, or none.
Outcomes. Comparison of pretreatment and posttreatment change in brain functioning and neural circuits through any neuroimaging modality (ie, functional magnetic resonance imaging [fMRI] or single-photon emission computed tomography [SPECT]) and/or comments on imaging biomarkers of response to psilocybin treatment.
Studies. Any original neuroimaging study, ie, randomized controlled trials (RCTs) or open-label studies.
Data Extraction and Analysis
Database search results were imported into the Covidence platform (https://www.covidence.org/) for deduplication, screening, and risk of bias assessment. Two reviewers (M.Y.J., S.Q.) independently screened the imported titles and abstracts and then assessed the remaining full texts for eligibility. Conflicts in judgment were resolved by discussion.
The data points to be extracted were determined a priori and included the following whenever possible: lead author, study type and duration, dosage and sequence of psilocybin administration, concomitant psychological intervention, modality of neuroimaging technique, baseline and posttreatment depression scores on validated psychological tools, whole and in-network brain functional changes, and correlation or association of brain functional changes with response to psilocybin treatment on any relevant clinical outcome (ie, decrease in depression, anxiety, or rumination symptomatology). A qualitative synthesis of the extracted data was subsequently undertaken.
Risk of Bias Assessment
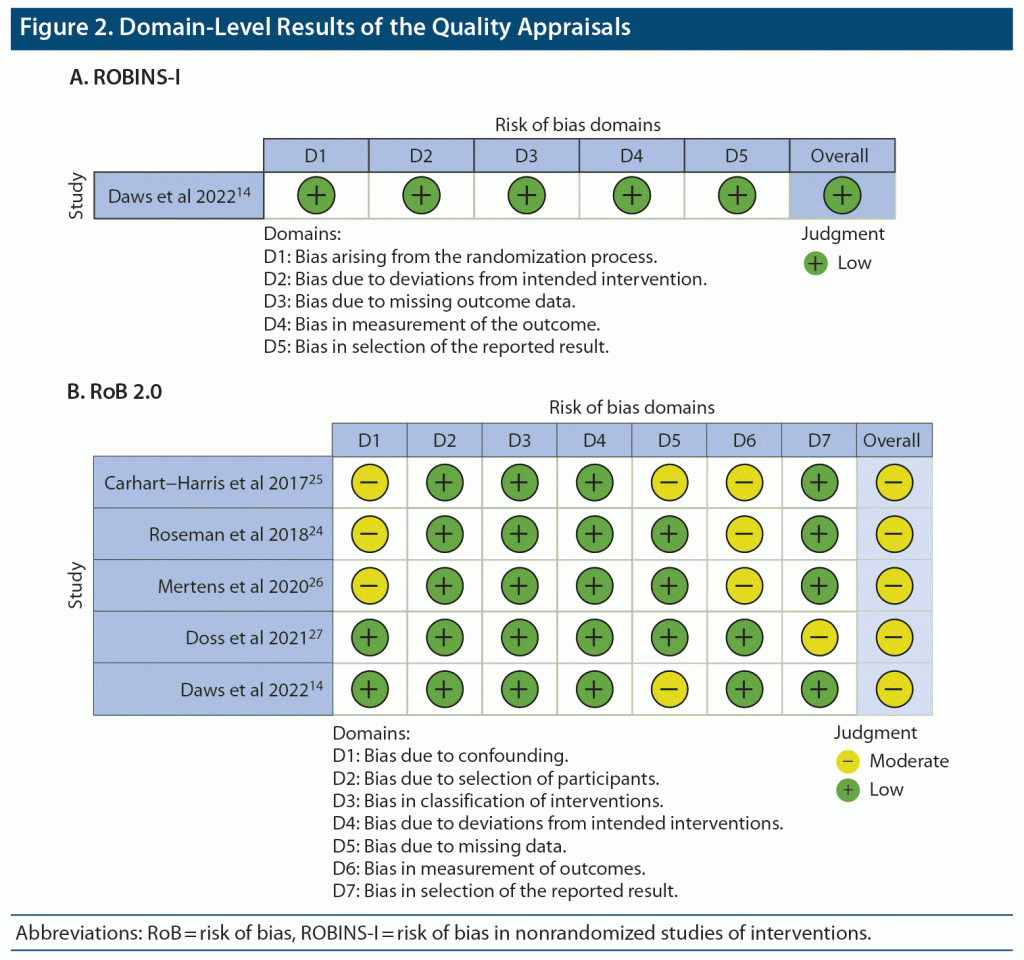
Assessments of methodological quality were independently conducted by 2 reviewers (S.Q. and Z.Z.) using Cochrane’s risk of bias tools.15 Conflicts in judgments were resolved by discussions that produced the consensus judgments reported herein. Since both blinded RCTs and unblinded open-label trials were included, 2 distinct tools were applied respectively: (1) the standard risk of bias (RoB) tool for RCTs and (2) the tool for risk of bias in non-randomized studies of interventions (ROBINS-I).
RESULTS
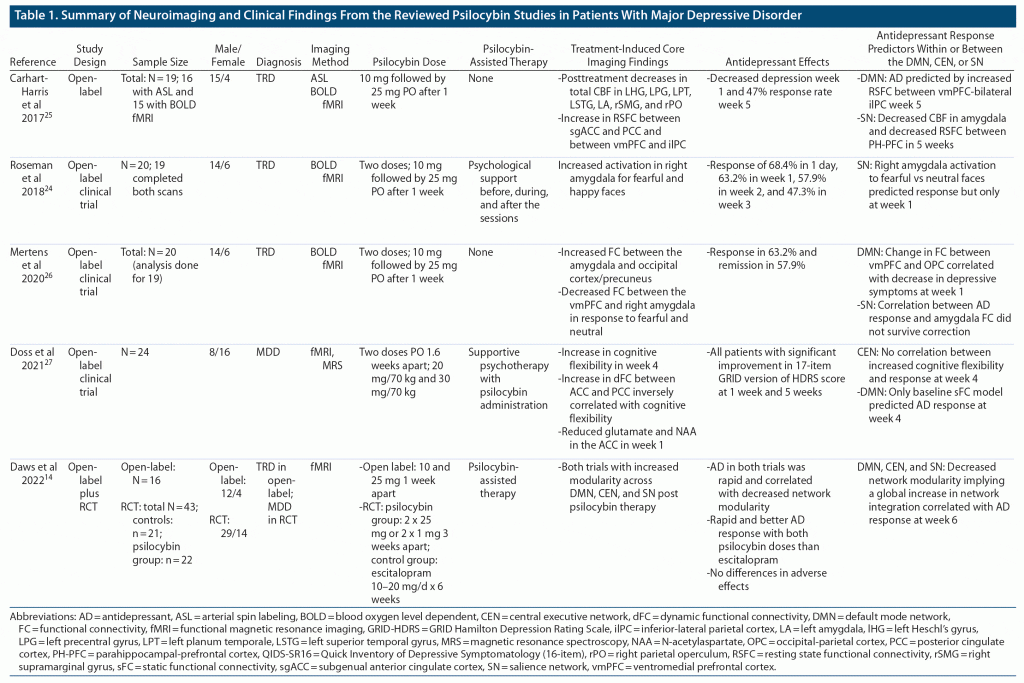
Of 946 studies identified in the initial search, 391 articles were left after duplicates were removed. These 391 studies underwent title and abstract screening, of which 8 studies were eligible for full-text analysis. After full-text research, 5 neuroimaging studies on depressed subjects were included in the narrative synthesis.14,24–27 As shown in Table 1, the first 4 studies were open-label trials without placebo control with a small sample size ranging from 14 to 24.24–27 However, 1 of the 2 trials in the latest study was an RCT with 43 subjects with MDD.14 All studies used functional magnetic resonance imaging (fMRI), with one also utilizing magnetic resonance spectroscopy to assess functional and biochemical changes in brain response to psilocybin, respectively.27 Four studies14,24–26 were conducted in patients with treatment-refractory depression except for the RCT from the latest study, which was completed in patients with major depression.14 Two studies24,26 examined the functional brain changes with psilocybin-assisted supportive psychotherapy. Psilocybin therapy produced a significant antidepressant response in all reviewed studies as assessed with the Quick Inventory for Depressive Symptoms28 or the Hamilton Depression Rating Scale.29
Study characteristics and extracted results are presented in Table 1. Furthermore, the ROBINS-I tool was applied to assess biases in selected open-label trials. Domain-level results of these quality appraisals are summarized in Figure 2. One limitation that needs to be mentioned here is that all studies were conducted in a single center and could have introduced any potential bias not measured by the ROBINS-I tool.
DISCUSSION
Although several neuroimaging studies with psilocybin are available in healthy volunteers, only a few are available in the depressed population, all of which are open-label and have utilized blood oxygen level–dependent (BOLD) fMRI (Table 1). Nevertheless, these psilocybin studies in depressed patients have reported interesting changes in the abnormal brain functioning reported in depressed patients.30,31 One of the most important brain networks affected in MDD is the default mode network (DMN)31 associated with self-referential processing.32 Several studies have reported increased activity in DMN,31 which explains excessive self-focus in depressed patients.33 Increased activity in DMN also dysregulates other higher-order brain networks, such as the central executive network (CEN) associated with cognitive inflexibility34 and the salience network (SN) associated with negative perceptions about self and the future.34,35 As depicted in Table 1, most neuroimaging findings from the reviewed studies involve changes in activation or functional connectivity within or between one or more of the specific brain regions in the DMN (ventromedial prefrontal cortex, posterior cingulate cortex, precuneus, and inferior lateral parietal cortex), CEN (dorsolateral prefrontal cortex and the lateral posterior parietal cortex), and SN (anterior cingulate cortex, amygdala, and parahippocampal gyrus).
The first study25 reported a greater decrease in resting-state functional connectivity (RSFC) in the parahippocampal–prefrontal cortex and a greater increase in the ventromedial prefrontal cortex–inferior lateral parietal cortex after a single 25-mg oral dose of psilocybin in responders than in the nonresponders. Both changes in psilocybin-induced connectivity predicted antidepressant response at 5 weeks. Increased connectivity in the ventromedial prefrontal cortex–inferior lateral parietal cortex reflected increased visuospatial ability to perceive self in the context of environment, while decrease in parahippocampal–prefrontal cortex connectivity represented prefrontal disinhibition allowing the spiritual experience. Although increased RSFC in the anterior cingulate cortex–posterior cingulate cortex/precuneus did not correlate with treatment response, increased connectivity between the anterior and posterior nodes of DMN may reflect a balance between emotionally laden self-perception versus other perspectives, respectively, and may help improve rumination and autobiographical memory in depressed patients. Other fMRI studies in depressed patients also found a significant relationship between a greater RSFC within the ventromedial prefrontal cortex and rumination scores in MDD, suggesting an inability to disengage DMN from self-perspective.36,37 It is worth mentioning that similar changes in DMN connectivity results have been reported in depressed subjects who responded to electroconvulsive therapy (ECT).38
Psilocybin-induced connectivity changes are transient and time dependent, and mood stabilization has been reported after a post-acute reduction in the DMN integrity with psilocybin25 and other psychedelics, such as LSD (lysergic acid diethylamide)39 and ayahuasca.40 The acute changes in connectivity are labeled as a “brain reset” mechanism as seen with ECT, in which initial disintegration facilitates a later reintegration and resumption of normal functioning. Of note, a concurrent reduction in BOLD activity has also been reported with a decrease in RSFC in posterior cingulate cortex/precuneus and medial prefrontal cortex with ayahuasca.40 In addition, the psilocybin-induced decoupling between anterior medial and posterior medial DMN was replicated in another study41 during a 5-day mindfulness retreat associated with altered self-perception and subjective ego dissolution.
The second study24 in depressed subjects, using an fMRI task for face recognition, reported a significant correlation between activation in the right amygdala to fearful and happy faces after a single dose of psilocybin lasting for 3 weeks. These findings contrast with the dampening of amygdala activity in response to negative emotional stimuli with conventional antidepressants, particularly SSRIs.14,25 These neuroimaging differences between psilocybin and SSRIs suggest entirely different mechanisms of emotional processing,14 where SSRIs reduce emotional responsiveness, resulting in emotional numbness,4 while psilocybin desensitizes patients to deal with painful emotions.24,42
However, psilocybin-induced amygdala activation is transient, and mood stabilization occurs after a reduction in amygdala activation. These findings resemble the acute connectivity changes reported with psilocybin25 and ECT.38,43 Psilocybin-induced brain reset in cerebral blood flow has also been reported in healthy volunteers.44,45 The acute disintegration followed by reintegration of neural circuits with psilocybin provides a brief but powerful window of opportunity for cognitive reframing, particularly with concurrent psychological support.24,26 Posttreatment increases in cerebral blood flow in the anterior cingulate cortex, medial prefrontal cortex, lateral prefrontal cortex, and medial temporal cortex have been associated with the hallucinatory ego disintegration in healthy subjects.46 Similar brain changes have been reported during ECT-induced seizures.38,43
The next study utilizing an fMRI face recognition task also reported acute changes in functional connectivity between important nodes of the DMN and amygdala in response to fearful and neutral faces after a week of psilocybin-assisted therapy.26 The most noticeable findings were a transient posttreatment reduction in right amygdala connectivity with ventromedial prefrontal cortex with increased amygdala connectivity with the visual cortex, including precuneus.26 Although the antidepressant response was not correlated with a reduction in ventromedial prefrontal cortex–right amygdala functional connectivity, rumination scores were significantly decreased at 1 week and 3 months after psilocybin treatment. These findings are consistent with results from earlier studies and support time-dependent changes behind brain reset with psilocybin therapy.
The next reviewed study27 reported an increase in psilocybin-induced cognitive and neural flexibility. The neural flexibility was expressed as the dynamic functional connectivity (dFC) between the anterior cingulate cortex and posterior cingulate cortex, a finding replicated from a previous study.25 However, none of the neuroimaging models predicted antidepressant response except the model trained on baseline static functional connectivity at 4 weeks. In addition, the reversal of the positive correlation between neural and cognitive flexibility after a week of psilocybin treatment suggests that sustained improvement in dFC may be counterproductive for cognitive flexibility. In other words, psilocybin therapy provides a transient window of opportunity for cognitive improvement, which closes after a sustained increase in dFC between the anterior cingulate cortex and posterior cingulate cortex. This is analogous to psilocybin-induced brain reset as reflected by disintegration and reintegration of neural circuits to deal with painful negative emotions. This study27 also reported a decrease in glutamate and N-acetylaspartate in the anterior cingulate cortex, which is counterintuitive as it reflects a decrease in neuronal metabolism or a deficit in white matter integrity. However, these changes may also reflect a transient brain reset. The latest psilocybin study14 in depressed subjects combined an open-label and a double-blind RCT of orally administered psilocybin in treatment-refractory and non–treatment-refractory patients, respectively. The second trial compared 2 doses of 25 mg of psilocybin therapy 3 weeks apart with daily 10–20 mg of escitalopram for 6 weeks. Both trials reported a rapid and durable response, which correlated with a lower strength of division (or modularity) between the DMN, CEN, and SN, suggesting a global integration in network activity. These network changes are most probably mediated by the psilocybin-induced activation of serotonin 2A (5-HT2A) receptors,47 most prominently expressed in the DMN, CEN, and SN.48 In contrast, antidepressant response with escitalopram was significantly lower than the psilocybin response and did not correlate with changes in any brain networks.14 The lack of selective activation of 5-HT2A receptors with SSRIs is perhaps the main reason escitalopram failed to show psilocybin-like changes in the brain networks.49 The findings from the first RCT carry more weight than the results from the open-label studies and support psilocybin’s global integration of important brain networks dysregulated during depression. The preliminary findings from this review should be interpreted with caution, as they are primarily based on open-label studies with small sample sizes. Only the large sample RCT can confirm the neuroimaging findings from the reviewed studies.
CONCLUSION
The neuroimaging findings from the reviewed studies have produced promising predictors of psilocybin response, which are uniquely different from neurobiological changes observed with conventional antidepressants. Psilocybin and other psychedelics are the only antidepressants after ECT with a positive correlation between a transient brain reset mechanism and antidepressant response, which can potentially revolutionize depression treatment. Future research should be conducted in large RCTs to replicate and confirm the results from the reviewed studies, particularly concerning the global integration of dysregulated brain networks underlying psilocybin’s response. In addition, the novel neurobiological effects of psilocybin justify transdiagnostic investigations, even including terminally ill patients and those with pervasive disorders such as autism and personality disorders. In addition, none of the studies reported any adverse effects with psilocybin therapy. The only adverse effects documented in the RCT were in the escitalopram group, wherein 4 of the 29 patients discontinued due to adverse reactions, but none of the 30 patients randomized to the psilocybin group discontinued the study 14. Most likely, there were no reportable adverse effects at the doses employed in the reviewed studies, which were much lower than those reported to result in a bad trip. In addition, no more than 2 psilocybin doses (10 mg and/or 25 mg) were used in all the reviewed studies, which could be another reason for the lack of adverse effects. However, a recent large sample trial15 reported headache, dizziness, and fatigue in the study subjects, with a few having serious adverse events including suicidal ideation and nonsuicidal self-injurious behavior. In addition, since depressed patients may have different genetic or neurobiological vulnerabilities, caution is warranted in using even the lower dose of psilocybin, particularly without psychotherapy and social support, until future research determines the safe and effective use of psilocybin in depressed patients.
Submitted: September 21, 2022; accepted December 30, 2022.
Published online: May 23, 2023.
Relevant financial relationships: None.
Funding/support: None.
ORCID: Saleha Qasim: 0000-0002-2311-8066; Zaofashan Zaheer: 0000-0001-7517-7140; Muhammad Youshay Jawad: 0000-0003-0997-8198; Mujeeb U Shad: 0000-0002-5136-9452
Clinical Points
- Psilocybin therapy appears promising in patients with treatment-refractory depression.
- Initial neuroimaging findings suggest an association between psilocybin-induced brain changes and antidepressant response.
- Future trials are required before psilocybin can be used safely and effectively in depressed patients.
References (49)

- McIntyre RS, Lee Y, Mansur RB. Treating to target in major depressive disorder: response to remission to functional recovery. CNS Spectr. 2015;20(suppl 1):17–30, quiz 31. PubMed CrossRef
- Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370–377. PubMed
- Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16–22. PubMed
- Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31–35. PubMed CrossRef
- Ochs-Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28(2):121–141. PubMed
- Seligman PJ, Anguiano RH, Felix T, et al. Managing the risks of medicines: an examination of FDA’s application of criteria for requiring a REMS. Ther Innov Regul Sci. 2019;53(4):542–548. PubMed CrossRef
- Heifets BD, Bentzley BS, Williams N, et al. Unraveling the opioid actions of S-ketamine and R-ketamine: comment on Bonaventura et al. Mol Psychiatry. 2021;26(11):6104–6106. PubMed CrossRef
- Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779–1786. PubMed CrossRef
- Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology. 2017;42(11):2105–2113. PubMed CrossRef
- Chi T, Gold JA. A review of emerging therapeutic potential of psychedelic drugs in the treatment of psychiatric illnesses. J Neurol Sci. 2020;411:116715. PubMed CrossRef
- Vargas AS, Luís Â, Barroso M, et al. Psilocybin as a new approach to treat depression and anxiety in the context of life-threatening diseases-a systematic review and meta-analysis of clinical trials. Biomedicines. 2020;8(9):331. PubMed CrossRef
- Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384(15):1402–1411. PubMed CrossRef
- Gukasyan N, Davis AK, Barrett FS, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J Psychopharmacol. 2022;36(2):151–158. PubMed CrossRef
- Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844–851. PubMed CrossRef
- Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387(18):1637–1648. PubMed CrossRef
- Sellers EM. Psilocybin: Good Trip or Bad Trip. Clin Pharmacol Ther. 2017;102(4):580–584. PubMed CrossRef
- Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603–620. PubMed CrossRef
- Bienemann B, Ruschel NS, Campos ML, et al. Self-reported negative outcomes of psilocybin users: a quantitative textual analysis. PLoS One. 2020;15(2):e0229067. PubMed CrossRef
- Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268–1278. PubMed CrossRef
- Krebs TS, Johansen PO. Psychedelics and mental health: a population study. PLoS One. 2013;8(8):e63972. PubMed CrossRef
- Johansen PO, Krebs TS. Psychedelics not linked to mental health problems or suicidal behavior: a population study. J Psychopharmacol. 2015;29(3):270–279. PubMed CrossRef
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. PubMed CrossRef
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74(9):790–799. PubMed CrossRef
- Roseman L, Demetriou L, Wall MB, et al. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology. 2018;142:263–269. PubMed CrossRef
- Carhart-Harris RL, Roseman L, Bolstridge M, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep. 2017;7(1):13187. PubMed CrossRef
- Mertens LJ, Wall MB, Roseman L, et al. Therapeutic mechanisms of psilocybin: changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J Psychopharmacol. 2020;34(2):167–180. PubMed CrossRef
- Doss MK, Považan M, Rosenberg MD, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry. 2021;11(1):574. PubMed CrossRef
- Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. PubMed CrossRef
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. PubMed CrossRef
- Goodman ZT, Bainter SA, Kornfeld S, et al. Whole-brain functional dynamics track depressive symptom severity. Cereb Cortex. 2021;31(11):4867–4876. PubMed CrossRef
- Hamilton JP, Furman DJ, Chang C, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. PubMed CrossRef
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316(1):29–52. PubMed CrossRef
- Lyons T, Carhart-Harris RL. More realistic forecasting of future life events after psilocybin for treatment-resistant depression. Front Psychol. 2018;9:1721. PubMed CrossRef
- Kim C, Cilles SE, Johnson NF, et al. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp. 2012;33(1):130–142. PubMed CrossRef
- Turnbull A, Karapanagiotidis T, Wang HT, et al. Reductions in task positive neural systems occur with the passage of time and are associated with changes in ongoing thought. Sci Rep. 2020;10(1):9912. PubMed CrossRef
- Berman MG, Peltier S, Nee DE, et al. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. PubMed CrossRef
- Hamilton JP, Farmer M, Fogelman P, et al. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78(4):224–230. PubMed CrossRef
- Mulders PC, van Eijndhoven PF, Pluijmen J, et al. Default mode network coherence in treatment-resistant major depressive disorder during electroconvulsive therapy. J Affect Disord. 2016;205:130–137. PubMed CrossRef
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A. 2016;113(17):4853–4858. PubMed CrossRef
- Palhano-Fontes F, Andrade KC, Tofoli LF, et al. The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS One. 2015;10(2):e0118143. PubMed CrossRef
- Smigielski L, Scheidegger M, Kometer M, et al. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage. 2019;196:207–215. PubMed CrossRef
- Carhart-Harris RL, Erritzoe D, Haijen E, et al. Psychedelics and connectedness. Psychopharmacology (Berl). 2018;235(2):547–550. PubMed CrossRef
- Pang Y, Wei Q, Zhao S, et al. Enhanced default mode network functional connectivity links with electroconvulsive therapy response in major depressive disorder. J Affect Disord. 2022;306:47–54. PubMed CrossRef
- Lewis CR, Preller KH, Kraehenmann R, et al. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage. 2017;159:70–78. PubMed CrossRef
- Vollenweider FX, Leenders KL, Oye I, et al. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol. 1997;7(1):25–38. PubMed CrossRef
- Carhart-Harris RL, Erritzoe D, Williams T, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012;109(6):2138–2143. PubMed CrossRef
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, et al. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9(17):3897–3902. PubMed CrossRef
- Beliveau V, Ozenne B, Strother S, et al. The structure of the serotonin system: a PET imaging study. Neuroimage. 2020;205:116240. PubMed CrossRef
- Preskorn S. Targeted pharmacotherapy in depression management: comparative pharmacokinetics of fluoxetine, paroxetine and sertraline. Int Clin Psychopharmacol. 1994;9(suppl 3):13–19. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite