Abstract
Objective: Major depressive disorder (MDD) is a common psychiatric disorder for which pharmacologic standard-of-care treatments have limited efficacy, particularly among individuals with cognitive dysfunction. Cognitive dysfunction is observed in approximately 25%–50% of those with MDD, wherein response to standard-of-care medications is reduced. Vortioxetine is an approved antidepressant that has shown evidence of procognitive effects in patients. It is not known if it has greater clinical efficacy in MDD patients with cognitive dysfunction, a more difficult to treat population, than other antidepressants.
Methods: This study was a reanalysis of 1,812 subjects with MDD across 4 placebo controlled trials. Baseline cognition was measured by the Digit Symbol Substitution Test (DSST), the primary measure used to demonstrate vortioxetine’s procognitive effects in clinical studies. Analyses examined whether baseline cognitive function was associated with differences in treatment outcomes.
Results: Baseline DSST did not predict placebo-adjusted treatment effects of vortioxetine on depressive symptoms (pooled Cohen d = −0.02, 95% CI = −0.12 to 0.07). Analyses of additional cognitive measures similarly did not predict placebo-adjusted treatment effects on depression (all 95% CI contained zero). Finally, analyses of trials with selective serotonin reuptake inhibitors (SSRIs)/serotonin and norepinephrine reuptake inhibitors (SNRIs) as active comparators also revealed no prediction of SSRI/SNRI adjusted treatment effects of vortioxetine on depression.
Conclusions: These findings, taken together, suggest that cognitive function does not moderate depression outcomes in vortioxetine, with results comparable to other antidepressants.
J Clin Psychiatry 2024;85(4):24m15295
Author affiliations are listed at the end of this article.
Major depressive disorder (MDD) is a common psychiatric disorder1 and a leading cause of disability worldwide.2 MDD, which often begins in early adulthood, has a high recurrence rate and is associated with premature death, including the highest risk for suicide among mental disorders.3 Antidepressants are widely used for the treatment of MDD, but there is considerable concern about their efficacy due to modest short-term benefits,4 with a drug placebo effect size that barely surpasses conventional criteria for a small effect.5 Given that MDD is a highly heterogeneous disorder, and clinical outcomes vary substantially among patients that receive the same treatment,6 predictive markers are needed to identify subpopulations of patients that respond or do not respond to specific interventions.7,8
Cognitive dysfunction is a core feature of MDD, in which heterogeneous deficits exist across multiple cognitive domains, including executive function, attention, processing speed, learning, and memory.9–11 Significant cognitive impairment (at least 1 SD below healthy normative samples) is observed in approximately 25%–50% of patients with MDD,12,13 with a subset of patients exhibiting levels of cognitive impairment frequently observed in schizophrenia.13 MDD patients with cognitive impairment experience poorer functional outcomes,14–16 delayed treatment response, increased risk for relapse,17 and increased risk for suicide.18 Cognitive dysfunction persists in remission and worsens with repeated episodes.19 Traditional monoamine-based antidepressants such as selective serotonin reuptake inhibitor (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) are less effective at treating depressive symptoms for patients with cognitive dysfunction than those who are cognitively intact,20,21 which suggests that antidepressants with a procognitive pharmacologic profile may be crucial for this particularly severe form of MDD.
Vortioxetine is an approved antidepressant with unique properties that may be well suited for this subset of MDD. Vortioxetine is a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5- HT1A receptor agonist and serotonin (5-HT) transporter inhibitor that increases serotonergic, noradrenergic, dopaminergic, cholinergic, histaminergic, and glutamatergic neurotransmission.22 This unique combination of mechanisms gives it antidepressant as well as procognitive properties. Indeed, preclinical research in rats observed that vortioxetine restored memory performance, while escitalopram and duloxetine had no such effect.23 As an antidepressant, vortioxetine yields effect sizes comparable to those of other antidepressants in treating depression symptoms in all-comer trials when compared with placebo.5,24 Additionally, in a meta-analysis of placebo-controlled trials, vortioxetine was the only antidepressant shown to improve executive functioning compared to placebo, as measured by tests of information processing speed/ working memory and cognitive flexibility.25 By contrast, both vortioxetine and duloxetine improved delayed verbal memory compared to placebo.25
Given that vortioxetine has demonstrated comparable efficacy to other antidepressants in the treatment of MDD, and superior efficacy over other antidepressants in improving cognitive performance, it raises the question as to whether vortioxetine may be more effective in treating depressive symptoms among those with cognitive dysfunction who do not have any specific treatment options. A similar logic with respect to whether preferential outcomes with a drug potentially informing which patients may be best suited for it has been successfully used in depression. For example, prior work has shown that aticaprant improves anhedonia symptoms,26 leading researchers to test whether its antidepressant effects are greater for patients with elevated anhedonia. A recent clinical trial suggests that this may indeed be the case.27 Similarly, prior work has shown that seltorexant is particularly effective in treating depression symptoms among those with elevated insomnia symptoms.28 Prior studies have suggested that changes in cognition and depressive symptoms occur independently of one another with vortioxetine29; however, no studies to date have examined whether cognitive function moderates depression outcomes in vortioxetine. If this is the case, then it would suggest that vortioxetine may be a preferable treatment of choice for individuals with MDD and cognitive dysfunction.
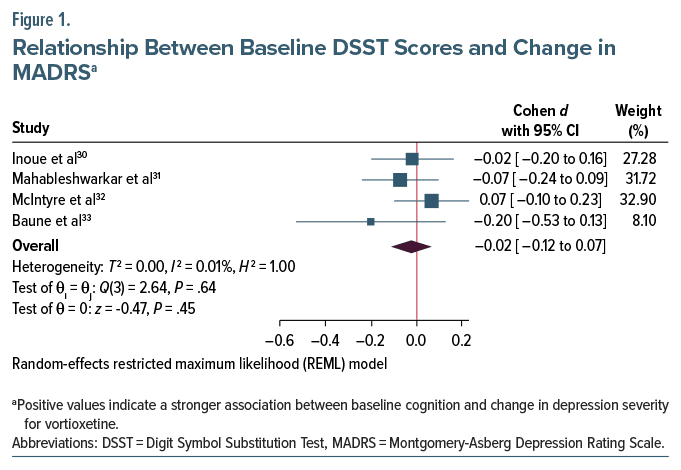
The current study is a reanalysis of several randomized trials of vortioxetine, all of which reported superiority of vortioxetine over placebo in treating depressive symptoms on the Montgomery-Asberg Depression Rating Scale (MADRS)30–33; 2 of the trials reported improvement in cognition over placebo,31,32 while 2 reported no difference.30,33 This study will evaluate whether baseline cognitive performance is associated with greater change in depression severity with vortioxetine compared to placebo and whether baseline cognitive performance is associated with greater change in depression severity with vortioxetine compared to active comparators.
METHODS
Participants
Data were analyzed from 4 randomized, double-blind, placebo-controlled trials (NCT01422213, NCT01564862, NCT02389816, and NCT02279966) in subjects who met criteria for recurrent MDD based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, criteria, confirmed by the Mini International Neuropsychiatric Interview, and had a MADRS total score of ≥22 at baseline. The Full Analysis Set (FAS) consisted of subjects who had taken at least 1 dose of study medication, provided at least 1 post baseline clinical assessment, had baseline cognitive data, and were ≤65 years old. One trial31 included duloxetine (60 mg/d) as an active-comparator group, while another33 included paroxetine (20 mg/d). Subjects assigned to vortioxetine were dosed at 10–20 mg/d30–32 and were pooled into 1 group. All studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practices guidelines and with the Declaration of Helsinki. All protocols, related forms, and amendments were approved by local research ethics committees. All subjects provided written informed consent before participating. Data were accessed through the Vivli platform34 after approval for secondary data analysis was granted by the trial sponsors.
Assessments
Full details of all assessments administered can be found in the original published manuscripts.30–32 For the present study, the MADRS was used as the primary outcome measure for all trials. We elected to use the Digit Symbol Substitution Test (DSST) (total correct) as the primary measure of cognition, although it is not a measure of global cognition. The DSST is a polyfactorial test that primarily assesses processing speed and attention, as well as executive function, associative learning, and working memory, all of which are profoundly impaired in MDD.29 It is highly sensitive to cognitive dysfunction and is correlated with functional outcomes.35 The DSST was chosen as the primary measure of cognition as it was administered in all of the included trials, was the primary cognitive outcome measure in previous trials of vortioxetine and cognition, and is the test in which the largest procognitive effects of vortioxetine are observed.29,36 Secondary analyses of other cognitive tests—including delayed memory, in which vortioxetine has also been shown to improve25—can be found in the Supplementary Material.
Statistical Analysis
Prior to analysis, cognitive variables were standardized to enhance clinical interpretability of performance and to adjust for demographic effects known to affect cognitive performance. Demographic information was limited for some trials as sponsors applied an additional layer of anonymization to ensure that the risk for patient identification was below 9%. Because of this, subject age was categorized in some trials into quartiles that were not universally consistent between studies, and we were unable to apply published norms to the data. Level of educational attainment was also not available. Because we were unable to convert cognitive scores to published normative data, cognitive performance was not binarized to classify subjects as either cognitively intact or impaired; instead, cognitive performance was treated as a continuous variable. In order to reduce the impact of demographic characteristics on cognitive tests, we stratified the samples based on age (<45 and ≥45, as this was the median categorical age group in some trials) and sex (male or female), and standardized cognitive variables by subtracting the sample mean from each subject’s individual score and dividing by the sample SD within each subject’s stratified demographic sample. This was done within each study to avoid ecological bias.37
In order to test the hypothesis that cognitive dysfunction at baseline is associated with greater treatment effects in vortioxetine, data were analyzed in 2 stages. First, to test the primary hypothesis, mixed models for repeated measures were conducted for each study, consistent with efficacy analyses used for each study.30–33 The FAS consisted of subjects who had taken at least 1 dose of study medication, provided at least 1 post-baseline assessment, and had baseline cognitive data. All models used change from baseline as the dependent variable (consistent with the prior studies) and included fixed effects of baseline MADRS, week (treated as categorical), baseline cognition (treated as continuous), and treatment arm; interaction terms included baseline severity × week, baseline cognition × week, treatment arm × week, baseline cognition × treatment arm, and baseline cognition × treatment arm × week. All models were estimated with restricted maximum likelihood and with the Kenward Roger adjustment for denominator degrees of freedom. Missing data were assumed to be missing at random; direct maximum likelihood was used to accommodate missing data. A single unstructured variance covariance matrix was used to model within-subject variation. The primary outcome of interest was the contrast in the relationship between baseline cognition and MADRS change between vortioxetine and placebo based on the interaction between baseline cognition and treatment at week 8. Estimates of the differences between slopes were extracted for each study. Effect size estimates were converted from t statistics for the contrast in slopes to an estimate of Cohen d, defined as 2t/√df , where t refers to the t statistic and df refers to the degrees of freedom.38 Because duloxetine and paroxetine were included as active comparators in 2 studies, we also examined the difference in the relationship between baseline cognition and MADRS change between duloxetine or paroxetine and placebo, as well as between duloxetine or paroxetine and vortioxetine.
Once estimates were obtained, random-effects models with restricted maximum likelihood were used to pool study results and provide weighted estimates along with their 95% CI. Due to the large sample size, statistical significance of the effect size was based on the 95% CI; if the 95% CI did not contain zero, the pooled effect was considered statistically significant. This study was powered to detect a very small effect size as low as d = 0.08, assuming 80% power, 2-sided P value of .05, regardless of the degree of heterogeneity.39
All analyses were conducted in Stata v17.040 and Rv4.2.2.41
RESULTS
Altogether, 1,812 subjects were included in analyses; 590 were assigned to placebo, 204 to duloxetine, 55 to paroxetine, and 963 to vortioxetine. There were 1,093 female subjects (60.3%); 794 (43.8%) of subjects were in the ≤45 group. There was an even distribution of sex between treatment arms across studies (all Pearson χ2 ≤ 2.28, P ≥ .319, Cramer V ≤ 0.06), and age was similarly evenly distributed across studies (all Pearson χ2 ≤ 3.30, P ≥ .07, Cramer V ≤ 0.08). There was no difference in baseline cognition between treatment arms (P ≥ .07, Cohen d ≤ 0.18) or baseline MADRS severity (P ≥ .155). The mean baseline MADRS severity was 31.33 (SD = 3.73, range = 22–49). There was a statistically significant relationship between baseline DSST and baseline MADRS severity across all studies (pooled Pearson r = −0.12, 95% CI, −0.20 to −0.05), such that worse performance on the DSST was associated with greater baseline depression severity.
Estimates of the contrast in slopes between vortioxetine and placebo can be found in Figure 1. There was no difference between vortioxetine and placebo in terms of the relationship between baseline cognition and with change from baseline on the MADRS at week 8 (pooled Cohen d = −0.02, 95% CI, −0.12 to 0.07). There was no heterogeneity between studies (I 2 ≤ 0.01%). Secondary analyses of other cognitive measures did not reveal a significant difference in slopes between vortioxetine and placebo across other cognitive tests (see Supplementary Material and Supplementary Figure 1).
In the data from Mahableshwarkar et al,31 the relationship between baseline cognition and change from baseline at week 8 on the MADRS was no different between vortioxetine and duloxetine (contrast = −1.61, t = −1.68, P = .094; d = −0.14, 95% CI, −0.31 to 0.02), and the contrast in slopes was not different in duloxetine and placebo (contrast = −0.76, t = −0.79, P = .430; d = −0.07, 95% CI, −0.10 to 0.23). Similarly, there was no difference in the relationship between baseline cognition and change from baseline at week 8 on the MADRS between vortioxetine and paroxetine (contrast = −0.45, t = −0.25, P = .801; d = −0.04, 95% CI, −0.37 to 0.29), and the contrast in slopes was not different in paroxetine and placebo (contrast = 1.81, t = 1.07, P = .288; d = 0.18, 95% CI, −0.15 to 0.51). Additional analyses of other cognitive measures similarly did not reveal a significant difference in slopes between vortioxetine and duloxetine or paroxetine across tests of executive function and attention; additionally, there were no significant differences in slopes between duloxetine or paroxetine and placebo across other cognitive measures (see Supplementary Material).
DISCUSSION
Cognitive dysfunction impacts up to half of those suffering from MDD at levels considered to be clinically significant12,13 and is not effectively treated by standard of care antidepressants.20,21 Vortioxetine, because of its unique pharmacologic profile and evidence of procognitive effects in MDD,25 may be a preferable treatment option for those suffering from cognitive dysfunction; however, no study has previously examined whether baseline cognitive functioning is associated with depression treatment outcomes in vortioxetine. The present study was a reanalysis of 4 randomized, placebo controlled trials30–32; 2 of which also included an active reference group (duloxetine or paroxetine). Findings from the present study suggest that baseline cognitive function does not moderate or predict the relationship between vortioxetine and depression outcomes, which suggests that selecting patients based on their baseline cognitive functioning may not improve depression in vortioxetine, unlike aticaprant (for depression with elevated symptoms of anhedonia27) and seltorexant (for depression with elevated symptoms of insomnia28).
Ultimately, these findings do not dispute vortioxetine’s effects in reducing depressive symptoms, which are well documented.5,24 It does, however, suggest that vortioxetine is not more efficacious than other standard of care antidepressants in the treatment of depressive symptoms in MDD with cognitive dysfunction and that the stratification of patients based on baseline cognitive performance with vortioxetine does not improve depression outcomes. The mechanisms of action for antidepressants, including vortioxetine, are generally based on the monoamine hypothesis. It has been proposed that the largest potential advances in the field may come from the utilization of mechanisms of action that are different from current first-line treatments,42 and monoamine-alternate hypotheses are needed to explain the latency or insufficient response to monoamine-based agents.43 Broadly speaking, neuroplasticity promoting- and neurogenesis-based hypotheses are an attractive and reasonable alternative to the monoamine hypothesis,43 and this may be particularly true for MDD with cognitive dysfunction. For example, animal work on ketamine, a rapid-acting antidepressant that inhibits N-methyl-D aspartate receptor function, has demonstrated an acute increase in synaptic efficiency,44 and there is some evidence that it may improve cognition in MDD,45 although findings to date have been inconsistent and somewhat confounded by its acute negative effects on cognition.46 Compounds that promote neurogenesis in the hippocampus may alleviate depressive symptoms more effectively and rapidly,47 as the inhibition of hippocampal neurogenesis is thought to be responsible for cognitive impairment in MDD.48,49 To date, the effects of proneurogenic or prosynaptic compounds on MDD with cognitive dysfunction have yet to be examined but warrant further investigation.
There are several limitations that should be considered. There was not enough information to apply normative data to put individual-level scores into context (ie, precise numerical age, years of education, or other estimates of premorbid cognitive function). We were therefore unable to formally classify subjects as cognitively impaired or preserved, nor are we able to determine the representativeness of cognitive function in these studies relative to the MDD population as a whole. Other important measures of functional and patient-reported outcomes were inconsistently measured across studies or not measured at all, which restricts our findings to only one aspect of clinical outcomes in depression, the clinician-rated MADRS. Similarly, other aspects of cognition not measured in these trials may predict response to treatment in vortioxetine, although the included studies administered a number of tests and covered neurocognitive domains that are sensitive to cognitive dysfunction in MDD, and the DSST is a well known test of broad cognitive functioning.35 Finally, the trials included in the present study are not necessarily fully representative of all trials of vortioxetine and cognitive functioning, and findings may therefore be influenced by selection bias.
In summary, the findings from this reanalysis of several placebo-controlled trials suggest that cognitive function does not moderate depression outcomes in vortioxetine, which suggests that vortioxetine may not necessarily be preferable for treating depressive symptoms in individuals with depression and cognitive impairment over and above other antidepressants. Antidepressants that operate through a different mechanism of action that enhance synaptic efficiency or promote neurogenesis need to be tested to see if they may be preferable first-line treatments for treating depressive symptoms in MDD with cognitive dysfunction.
Article Information
Published Online: September 4, 2024. https://doi.org/10.4088/JCP.24m15295
© 2024 Physicians Postgraduate Press, Inc.
Submitted: February 7, 2024; accepted July 3, 2024.
To Cite: Jordan JT, Shen L, Cooper NJ, et al. Baseline cognition is not associated with depression outcomes in vortioxetine for major depressive disorder: findings from placebo-controlled trials. J Clin Psychiatry. 2024;85(4):24m15295.
Author Affiliations: Alto Neuroscience Inc Los Altos, California (Jordan, Shen, Cooper, Goncalves, Wu, Savitz, Etkin); Center for Depression Research and Clinical Care, Peter O’Donnell Jr Brain Institute and Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, Texas (Trivedi); Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California (Schatzberg, Etkin).
Corresponding Author: Amit Etkin, MD, PhD, Alto Neuroscience Inc, 650 Castro St, Ste 450, Mountain View, Los Altos, CA 94041 ([email protected]).
Author Contributions: Contributed to study conception and design and manuscript composition: (Jordan, Savitz, Etkin); performed the research and analyzed the data: (Jordan, Shen); wrote the manuscript with input from all other authors: (Jordan); contributed to data interpretation, manuscript composition, and editing: (Cooper, Goncalves, Wu, Trivedi, Schatzberg); contributed to data interpretation: (Trivedi, Schatzberg, Savitz, Etkin).
Relevant Financial Relationships: Drs Jordan, Shen, Cooper, Wu, Savitz, and Etkin and Ms Goncalves are employees of Alto Neuroscience and receive salary and equity. Dr Trivedi has provided consulting services to Acadia Pharmaceuticals, Alkermes Inc, Alto Neuroscience Inc, Axsome Therapeutics, Biogen MA Inc, Cerebral Inc, Circular Genomics Inc, Compass Pathfinder Ltd, GH Research, GreenLight VitalSign6 Inc, Heading Health, Janssen Pharmaceutical, Legion Health, Merck Sharp & Dohme Corp, Mind Medicine Inc, Myriad Neuroscience, Naki Health Ltd, Navitor, Neurocrine Biosciences Inc, Noema Pharma AG, Orexo US Inc, Otsuka America Pharmaceutical Inc, Otsuka Pharmaceutical Development & Commercialization Inc, Otsuka Pharmaceutical Europe Ltd, Perception Neuroscience Holdings, Pharmerit International, Policy Analysis Inc, Praxis Precision Medicines Inc, PureTech LYT Inc, Relmada Therapeutics Inc, Rexahn Pharmaceuticals, Inc, SAGE Therapeutics, Signant Health, Sparian Biosciences, Titan Pharmaceuticals, Takeda Pharmaceuticals Inc, and WebMD. He has received grant/research funding from National Institute of Mental Health, National Institute on Drug Abuse, National Center for Advancing Translational Sciences, American Foundation for Suicide Prevention, Patient-Centered Outcomes Research Institute (PCORI), and Blue Cross Blue Shield of Texas. Additionally, he has received editorial compensation from Engage Health Media, and Oxford University Press. Dr Schatzberg has served as a consultant to Alto Neuroscience, ANeurotech, Compass, Douglas, Magnus, NeuraWell, Parexel, Sage, and Signant. He holds equity in Alto Neuroscience, Corcept, Delpor, Madrigal, Magnus, Seattle Genetics, Titan, and Xhale. He receives salary and equity from Alto Neuroscience and has equity in Johnson & Johnson. Dr Etkin receives salary and equity from Alto Neuroscience and holds equity in Akili Interactive.
Funding/Support: This work was conducted by and supported by Alto Neuroscience Inc, Los Altos, California. No supporting organization was involved in the study planning, design, implementation, or reporting.
Acknowledgments: This publication is based on research using data from Takeda Pharmaceuticals and Lundbeck that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Individuals with depression and poor cognitive function are less likely to respond to antidepressants.
- Vortioxetine is an antidepressant that has unique procognitive properties, and baseline cognitive functioning may moderate depression outcomes in vortioxetine.
- Cognitive functioning at baseline was not found to moderate depression outcomes in those treated with vortioxetine across multiple studies. There was no evidence for improved depression outcomes in vortioxetine based on cognitive functioning.
References (49)

- Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. PubMed
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–150. PubMed CrossRef
- Moitra M, Santomauro D, Degenhardt L, et al. Estimating the risk of suicide associated with mental disorders: a systematic review and meta-regression analysis. J Psychiatr Res. 2021;137:242–249. PubMed CrossRef
- Ioannidis JPA. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philos Ethics Humanit Med. 2008;3:14. PubMed CrossRef
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. PubMed CrossRef
- Arnow BA, Blasey C, Williams LM, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D trial. Am J Psychiatry. 2015;172(8):743–750. PubMed CrossRef
- Saveanu R, Etkin A, Duchemin AM, et al. The international study to predict optimized treatment in depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. 2015;61:1–12. PubMed CrossRef
- Trivedi MH, McGrath PJ, Fava M, et al. Establishing moderators and biosignatures of antidepressant response in clinical care (embarc): rationale and design. J Psychiatr Res. 2016;78:11–23. PubMed CrossRef
- Bora E, Harrison BJ, Yücel M, et al. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–2026. PubMed CrossRef
- Parkinson WL, Rehman Y, Rathbone M, et al. Performances on individual neurocognitive tests by people experiencing a current major depression episode: a systematic review and meta-analysis. J Affect Disord. 2020;276:249–259. PubMed CrossRef
- Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. PubMed CrossRef
- Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69(7):1122–1130. PubMed CrossRef
- Pu S, Noda T, Setoyama S, et al. Empirical evidence for discrete neurocognitive subgroups in patients with non-psychotic major depressive disorder: clinical implications. Psychol Med. 2018;48(16):2717–2729. PubMed CrossRef
- Knight MJ, Air T, Baune BT. The role of cognitive impairment in psychosocial functioning in remitted depression. J Affect Disord. 2018;235:129–134. PubMed CrossRef
- Knight MJ, Lyrtzis E, Baune BT. The association of cognitive deficits with mental and physical Quality of Life in Major Depressive Disorder. Compr Psychiatry. 2020;97:152147. PubMed CrossRef
- Lam RW, Kennedy SH, Mclntyre RS, et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649–654. PubMed CrossRef
- Majer M, Ising M, Künzel H, et al. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med. 2004;34(8):1453–1463. PubMed CrossRef
- Keilp JG, Gorlyn M, Russell M, et al. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2013;43(3):539–551. PubMed CrossRef
- Semkovska M, Quinlivan L, O’Grady T, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(10):851–861. PubMed CrossRef
- Etkin A, Patenaude B, Song YJC, et al. A cognitive–emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology. 2015;40(6):1332–1342. PubMed CrossRef
- Groves SJ, Douglas KM, Porter RJ. A systematic review of cognitive predictors of treatment outcome in major depression. Front Psychiatry. 2018;9:382. PubMed
- Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57. PubMed CrossRef
- Jensen JB, du Jardin KG, Song D, et al. Vortioxetine, but not escitalopram or duloxetine, reverses memory impairment induced by central 5-HT depletion in rats: evidence for direct 5-HT receptor modulation. Eur Neuropsychopharmacol. 2014;24(1):148–159. PubMed CrossRef
- Zhang X, Cai Y, Hu X, et al. Systematic review and meta-analysis of vortioxetine for the treatment of major depressive disorder in adults. Front Psychiatry. 2022;13:922648. PubMed CrossRef
- Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. 2015;19(2):pyv082. PubMed
- Krystal AD, Pizzagalli DA, Smoski M, et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med. 2020;26(5):760–768. PubMed CrossRef
- Schmidt ME, Kezic I, Popova V, et al. Efficacy and safety of aticaprant, a kappa receptor antagonist, adjunctive to oral SSRI/SNRI antidepressant in major depressive disorder: results of a phase 2 randomized, double-blind, placebo controlled study. Neuropsychopharmacology. 2024;49(9):1437–1447. PubMed CrossRef
- Savitz A, Wajs E, Zhang Y, et al. Efficacy and safety of seltorexant as adjunctive therapy in major depressive disorder: a phase 2b, randomized, placebo controlled, adaptive dose-finding study. Int J Neuropsychopharmacol. 2021;24(12):965–976. PubMed CrossRef
- McIntyre RS, Harrison J, Loft H, et al. The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(10):pyw055. PubMed
- Inoue T, Sasai K, Kitagawa T, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and safety of vortioxetine in Japanese patients with major depressive disorder. Psychiatry Clin Neurosci. 2020;74(2):140–148. PubMed CrossRef
- Mahableshwarkar AR, Zajecka J, Jacobson W, et al. A randomized, placebo controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025–2037. PubMed CrossRef
- McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557–1567. PubMed CrossRef
- Baune BT, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive performance in working patients with major depressive disorder: a short-term, randomized, double-blind, exploratory study. J Affect Disord. 2018;229:421–428. PubMed CrossRef
- Bierer BE, Li R, Barnes M, et al. A global, neutral platform for sharing trial data. N Engl J Med. 2016;374(25):2411–2413. PubMed CrossRef
- Jaeger J. Digit Symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513–519. PubMed CrossRef
- Baune BT, Brignone M, Larsen KG. A network meta-analysis comparing effects of various antidepressant classes on the digit symbol substitution test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol. 2018;21(2):97–107. PubMed
- Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one stage and two-stage approaches, and why they may differ. Stat Med. 2017;36(5):855–875. PubMed CrossRef
- Ben-Shachar MS, Lüdecke D, Makowski D. Effectsize: estimation of effect size indices and standardized parameters. J Open Source Softw. 2020;5(56):2815.
- Harrer M, Cuijpers P, Furukawa T, et al. Dmetar: companion R package for the guide doing meta-analysis in R. 2019. http://dmetar.protectlab.org/
- StataCorp. Stata Statistical Software: Release 17. StataCorp LLC; 2021.
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2022. https://www.R-project.org/.
- Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971–975. PubMed CrossRef
- Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3–12. PubMed CrossRef
- Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801–811. PubMed CrossRef
- Gill H, Gill B, Rodrigues NB, et al. The effects of ketamine on cognition in treatment-resistant depression: a systematic review and priority avenues for future research. Neurosci Biobehav Rev. 2021;120:78–85. PubMed CrossRef
- Zhornitsky S, Tourjman V, Pelletier J, et al. Acute effects of ketamine and esketamine on cognition in healthy subjects: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2022;118:110575. PubMed CrossRef
- Malberg JE, Schechter LE. Increasing hippocampal neurogenesis: a novel mechanism for antidepressant drugs. Curr Pharm Des. 2005;11(2):145–155. PubMed CrossRef
- Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility–linking memory and mood. Nat Rev Neurosci. 2017;18(6):335–346. PubMed CrossRef
- Yun S, Reynolds RP, Masiulis I, et al. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat Med. 2016;22(11):1239–1247. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite