Abstract
Background: Insomnia is a common sleep disorder, associated with multiple health concerns. Current medications for insomnia are associated with higher safety risks if clinical practice guidelines or monograph recommendations are not followed. This study aims to understand real-world prescribing practices among patients with insomnia in Canada, including medication utilization, potentially inappropriate medication use, cost incurred, and lines of treatment.
Methods: This retrospective observational study utilized longitudinal drug claims data from 2018 to 2020 from the Canadian IQVIA National Private Drug Plan and Ontario Drug Benefit databases. Patients with any claims for medications approved for insomnia in Canada were identified. Four types of inappropriate medication usage were defined: (1) elevated daily dose; (2) extended duration of use for benzodiazepines (BZD) and/or Z-drugs; (3) combination use; and (4) opioid overlap with BZD and/or Z-drugs.
Results: In 2019, 597,222 patients with insomnia were identified; 64% were female, with an average age of 55 years. Inappropriate medication use was noted in 52.5% of adult patients (aged 18–65 years) and 69.5% of senior patients (aged >65 years). Extended duration was the most common inappropriate medication usage category. The annual cost of medications for insomnia was $54.8 million, and $30.3 million (55.2%) met inappropriate medication use criteria.
Conclusion: High prevalence of inappropriate medications usage in insomnia raises serious safety concerns for patients suffering from insomnia, particularly seniors, while also placing a substantial burden on the Canadian public and private health systems. This highlights an unmet need for better education regarding current guidelines and more effective and safer treatment options.
J Clin Psychiatry 2024;85(4):23m15015
Author affiliations are listed at the end of this article.
Insomnia is a sleep disorder characterized by difficulties in initiating or maintaining sleep and is recognized as a common public health issue globally.1,2 In Canada, it affects 9.5%–13.4%, predominantly middle-aged women.3–5 Persistent insomnia impacts the quality of life, increases the risk of disease,6,7 increases the economic burden,8 and is associated with increased mortality.9 Direct and indirect health care costs associated with insomnia in North America can reach $100 billion per year, which is 26%–46% higher than health care costs in those who do not have insomnia.10,11 The high prevalence of insomnia among COVID-19 patients and its strong association with depression, posttraumatic stress disorder, anxiety, and psychological distress have further increased the health care burden.12–16
Cognitive behavioral therapy for insomnia (CBT-I) is recommended as the primary intervention for insomnia, and pharmacotherapy can be considered alone or in combination with CBT-I.17–19 There are several pharmacologic interventions approved by Health Canada, namely, dual orexin receptor antagonists (lemborexant, suvorexant, and daridorexant; DORA) and nonbenzodiazepines (zopiclone, eszopiclone, zaleplon, and zolpidem; Z-drugs). Lemborexant and daridorexant were approved by Health Canada in November 2020 and April 2023, respectively.20,21 Suvorexant was approved in 2018, but not launched and subsequently removed from the market by the manufacturer in 2020 due to business reasons.22 In addition, 3 other classes, benzodiazepines (BZD), antidepressants, and antipsychotic medications, are used on-label and off-label for patients with insomnia.23 Sedative-hypnotics such as BZD and Z-drugs bind to γ-aminobutyric acid receptors and are associated with safety concerns, such as complex motor behaviors, an increased risk of respiratory depression, abuse potential, risk of fall, withdrawal syndrome, rebound insomnia, and tolerance.24 Accordingly, Health Canada and the US Food and Drug Administration have updated the safety warnings of BZD and Z-drugs patients, recommending that health care professionals limit the use of these drugs and reinforcing the risk of the combination use of opioid and BZD or other central nervous system (CNS) medications.25,26 In Canada, drug prescriptions are mostly dispensed at community pharmacies, and physician prescribing practice is consistent across provinces.27 A Canadian study showed a higher BZD dispensing rate in seniors,28 and 75% of new patients’ BZD prescriptions were initiated by family physicians.29
Despite the existence of treatment guidelines,30–32 there is significant inappropriate medication usage of insomnia treatment, such as higher-than-recommended doses of sedative-hypnotics, long-term use of sedative hypnotics, concurrent use with opioids, and combination use of CNS-active drugs. Several studies have shown that this can cause serious harm such as falls and fractures and increased risk of death, to patients affected by insomnia.24 Chronic use of sedative-hypnotics is associated with worse clinical outcomes and adverse events, especially in older adults,33 and there is little evidence to prove the efficacy and safety of sedative hypnotics as long-term treatment options for patients with insomnia.34 In addition to the adverse health outcomes, the associated health care cost is also higher due to the inappropriate medication usage.35 Therefore, managing inappropriate drug usage could reduce adverse events, which may help reduce the burden on health care systems, as well as optimize the use of health care resources.
There is paucity of information regarding real-world treatment patterns for insomnia in Canada. The primary objective of this study was to describe the real-world utilization of medications to treat insomnia and the costs incurred, focusing on inappropriate medication usage among patients in Canada. The secondary objective was to understand the lines of treatment among patients with insomnia in Canada.
METHODS
Data Source
This retrospective observational study used private and public administrative claims data from the Canadian IQVIA National Private Drug Plan (PDP) and Ontario Drug Benefit (ODB) databases. IQVIA PDP is the largest private drug plan claim database in Canada and is composed of private insurance carriers, third-party administrators, and benefit plan managers. PDP data capture approximately 80% of all pay-direct private drug plan claims in Canada, with over 12 million active claimants and over 129 million drug claims. The ODB database contains 100% of the fully adjudicated publicly funded prescription claims from Ontario. The ODB program covers most of the drug costs for patients aged ≥65 years, patients aged ≤24 years, and other eligible populations including individuals in long-term care home or on social assistance.36
These databases provide information of drugs dispensed in nonhospital setting related to patient demographics, drugs dispensed, quantity dispensed, days supplied, service date, pharmacy location, cost, and the specialty of the prescribing physician. Diagnosis information is not available; therefore, indication is inferred based on drug claim history and physician specialty. Ethics approval was not required for this study.
Study Design
For the primary objective, a cross-sectional study was conducted to assess utilization in each analysis year over a 3-year period from 1 January 2018 to 31 December 2020 (Figure 1). For the secondary objective of lines of treatment analysis, a longitudinal study was conducted to track patients’ progress through various treatment options over time. The lines of treatment refer to the sequence of different treatments patients received during the analysis period (Supplementary Methods and Supplementary Figure 1). Each time there was a change in existing treatment, it is considered a new “line” of treatment and counted towards the overall count of treatment lines.
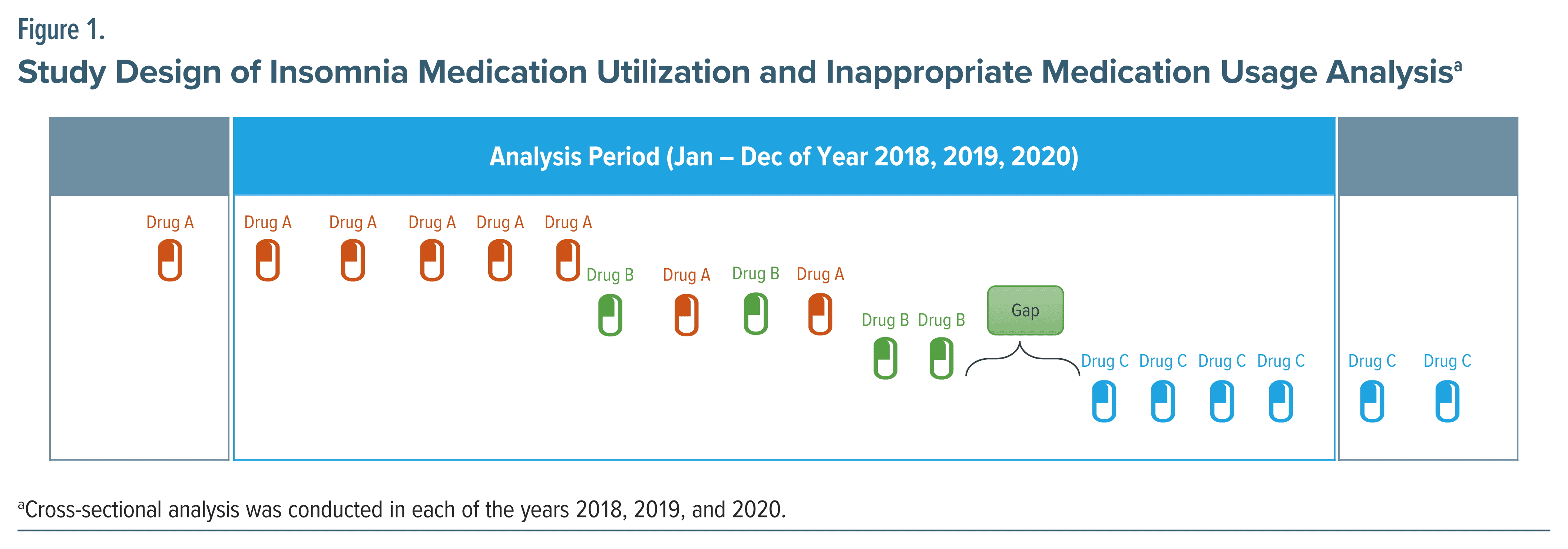 Five medication classes, DORA (only lemborexant), Z-drugs, BZD drugs, antidepressant drugs, and antipsychotic drugs, were defined as interventions for insomnia based on the Canadian Agency for Drugs and Technologies in Health.37 Of note, daridorexant was excluded from this study, as it was approved after the study period. Suvorexant was excluded due to low sample size.
Five medication classes, DORA (only lemborexant), Z-drugs, BZD drugs, antidepressant drugs, and antipsychotic drugs, were defined as interventions for insomnia based on the Canadian Agency for Drugs and Technologies in Health.37 Of note, daridorexant was excluded from this study, as it was approved after the study period. Suvorexant was excluded due to low sample size.
Study Population
In this study, patients who had at least 1 claim of lemborexant and/or Z-drugs within their full history were inferred as “patients with insomnia.” Only lemborexant and Z-drugs were used to infer patients with insomnia because they are only indicated for insomnia,38,39 while other classes can be used on-label or off-label for insomnia and/or other indications. For the primary objective, patients with insomnia who were aged ≥18 years, active in the plan, and had at least 1 claim of defined intervention for insomnia within the analysis year were selected. Selected patients were further grouped by age (adult patients: 18–65 years; senior patients: >65 years) and by payer type (PDP patients; ODB patients). For the secondary objective, patients with insomnia who were aged ≥18 years at the first claim of defined intervention for insomnia, active during the study period, and had at least 1 claim of defined intervention for insomnia during the selection period were selected.
Study Outcomes
In the primary objective, patient demographic characteristics, insomnia medications claimed, duration of supply per patient, prescribing daily dose per patient, daily units per patient, and drug cost per patient were reported. Within demographic characteristics, polypharmacy was defined as the count of distinct medication classes (Anatomical Therapeutic Chemical [ATC] level 2) that patients received during each analysis year. This includes all medications including those unrelated to insomnia. Patients may or may not have received the medications simultaneously. For the inappropriate medication usage analysis, patients with inappropriate medication usage and total cost on inappropriate medication usage were reported. In the secondary objective, patient demographic characteristics, treatment history per patient, and treatment lines were reported. A detailed description of the study outcomes is provided in Supplementary Table 1.
In the inappropriate medication usage analysis, 4 different types of inappropriate medication usage were defined as follows: (1) elevated daily dose was defined as patients exceeding the recommended maximum daily dose, as per the product monograph; (2) extended duration was defined as patients taking BZD and/or Z-drugs for a >30-day duration of supply, per the product monograph and therapeutic guidelines30,31; (3) combination use was defined as adult patients (18–65 years) who received a ≥1-day combination of any drug molecules within BZD and/or Z-drug; seniors (>65 years) who received a ≥1-day combination of any of the defined medications within the BZD, Z-drugs, antidepressants, and/or antipsychotics classes, as per therapeutic guidelines; and (4) opioid overlap was defined as patients taking BZD and/or Z-drugs overlapping with consecutive claims (≤7-day gap between claims) of opioid drugs for >30 days, as per the product monograph and therapeutic guidelines.31 The number of patients with each type of inappropriate medication usage, duration, and total cost of inappropriate usage were calculated in each analysis year.
Statistical Analyses
Descriptive statistics were reported as mean (SD) for continuous variables, and counts and proportions for categorical variables. Comparison between groups is numerical only as this is a descriptive study. Data management and data analysis were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Medication Utilization and Inappropriate Medication Usage
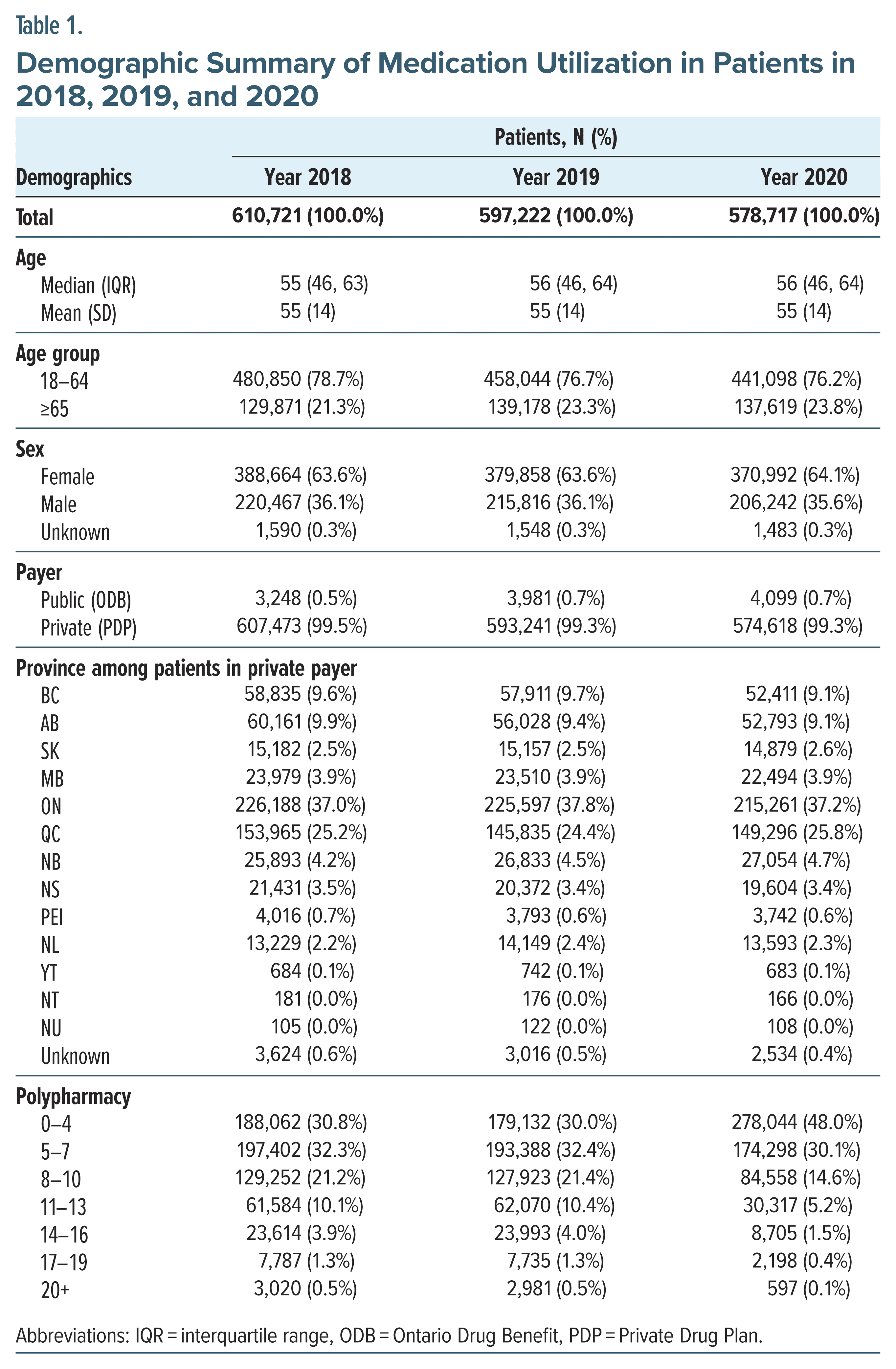
Demographic characteristics. In 2018, 2019, and 2020, a total of 610,721, 597,222, and 578,717 patients received defined interventions of insomnia, respectively. The majority (64%) were female, and over 70% were 18–64 years of age. Over 37% of the patients resided in Ontario, followed by 25% in Quebec, and 99% of the patients were from the PDP database. The majority of patients exhibited polypharmacy of 5 or more drug classes (ATC level 2) in 2018 (69.2%), 2019 (70.0%), and 2020 (52%). The demographic and baseline characteristics of the patient population were comparable across years (Table 1).
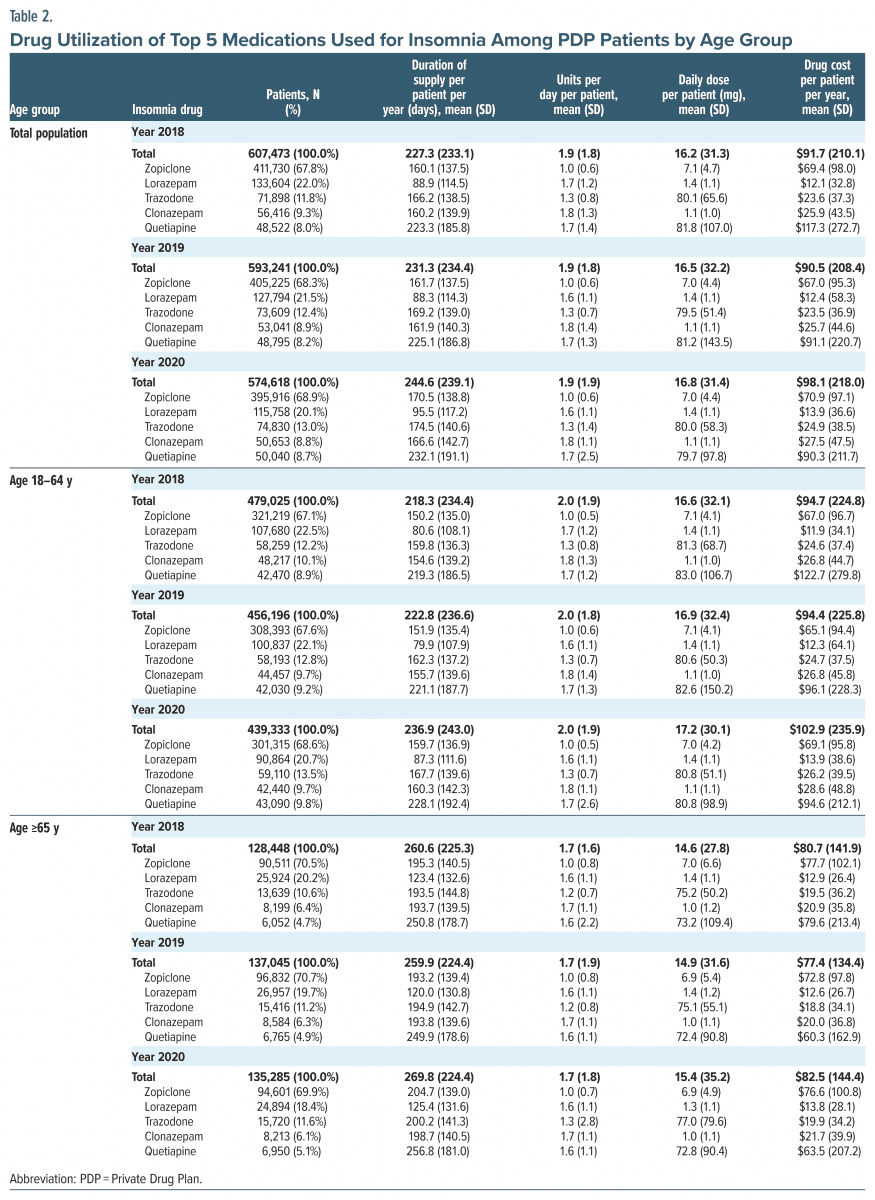
Medication utilization. The medication utilization followed similar trends between 2018 and 2020. Patients with 1 type of insomnia medication were more common in PDP compared to the ODB (66.7% vs 38.3% in 2019). There were more seniors who claimed only 1 type of insomnia medication than adult patients (70.5% vs 65.5% in 2019 in PDP and 48.3% vs 26.8% in 2019 on ODB) (Supplementary Table 2). The top 5 medications used for insomnia were zopiclone (Z-drug), lorazepam (BZD), trazodone (antidepressant), clonazepam (BZD), and quetiapine (antipsychotic) (Table 2).
Overall, the ODB patients had longer duration of supply, higher prescribed daily dose, and higher drug cost spent on medications for insomnia (Table 2; Supplementary Table 3). In 2019, the average annual drug cost for insomnia medications was $90.5 for PDP patients, compared to $283.2 for ODB patients. Among PDP patients, senior patients had approximately a month’s longer duration of supply, a lower prescribed daily dose, and a lower drug cost spent on medications compared to adult patients. In 2019, senior patients received medications for an average of 259.9 days’ supply, with the annual drug cost being $77.4 per patient, whereas adult patients received medications for an average of 222.8 days’ supply and a $94.4 cost (Table 2). The utilization of the top 5 medications is also listed in Table 2.
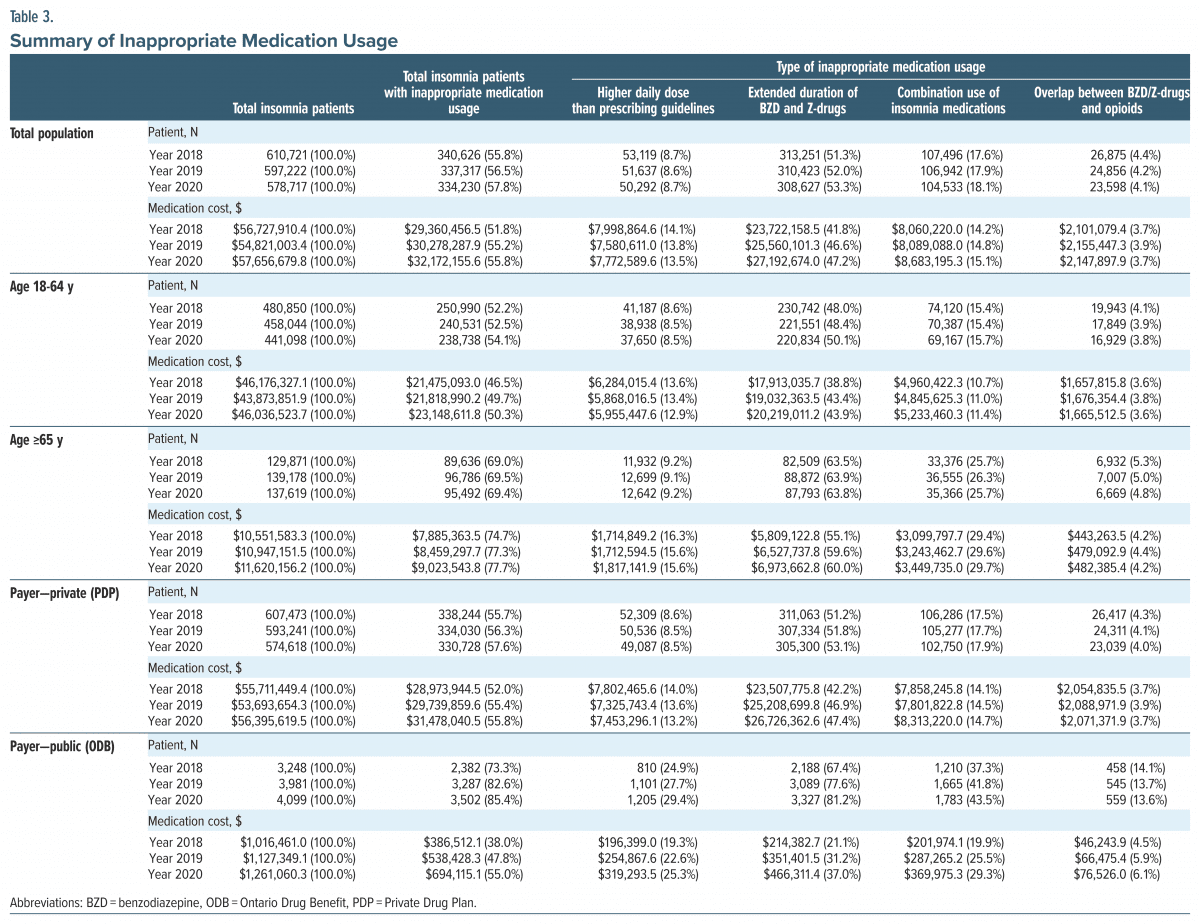
Inappropriate medication usage. Inappropriate medication usage for insomnia increased numerically over the 3 years (2018–2020). The total cost of all insomnia drugs in 2018 was $56.7 million, with 51.8% attributed to inappropriate drug usage ($54.8 million and 55.2% in 2019; $57.7 million and 55.8% in 2020). Between 2018 and 2020, the cost slightly increased among adult and senior patients (Table 3).
Extended duration of BZD and Z-drug use was the most frequent form of inappropriate medication usage, with the highest proportion of patients and cost of inappropriate medication usage (52.0% and 46.6% in 2019). The overlap between BZD/Z-drugs and opioids was less frequent (4.4% and 3.7% in 2018, 4.2% and 3.9% in 2019, and 4.1% and 3.7% in 2020) (Table 3).
Overall, a higher proportion of senior patients compared to adult patients showed inappropriate medication usage across all the 3 years (69.0% vs 52.2% in 2018, 69.5% vs 52.5% in 2019, and 69.4% vs 54.1% in 2020). Correspondingly, senior patients had a higher proportion of cost for inappropriate usage than adult patients (74.7% vs 46.5% in 2018, 77.3% vs 49.7% in 2019, and 77.7% vs 50.3% in 2020) (Table 3). The percentage of the cost of medication due to overlap between BZD/Z-drugs and opioids in adult and senior populations was 3.6% and 4.2% in 2018, 3.8% and 4.4% in 2019, and 3.6% and 4.2% in 2020. ODB patients had higher inappropriate usage than PDP patients (73.3% vs 55.7% in 2018, 82.6 vs 56.3% in 2019, and 85.4% vs 57.6% in 2020). The total cost of inappropriate medication usage was lower in ODB patients than in PDP patients (47.8% vs 55.4% in 2019) (Table 3).
Lines of Treatment
Demographic characteristics. A total of 240,820 patients met our selection criteria (study population). The majority were female (n = 148,689, 61.7%). Adult patients (18–64 years) comprised 81.8% of the cohort, with a mean age of 54 years. 99% of patients were from the PDP database, and 91,791 resided in the Ontario region (38.1%), while 61,451 (25.5%) were residents of Quebec (Supplementary Table 4).
Treatment history. The median treatment history from patients’ first drug claim for insomnia to the most recent claim was 3.5 years (interquartile range: 0.7, 8.0). Mean treatment history was 5.1 years (SD 5.2) (Supplementary Table 5).
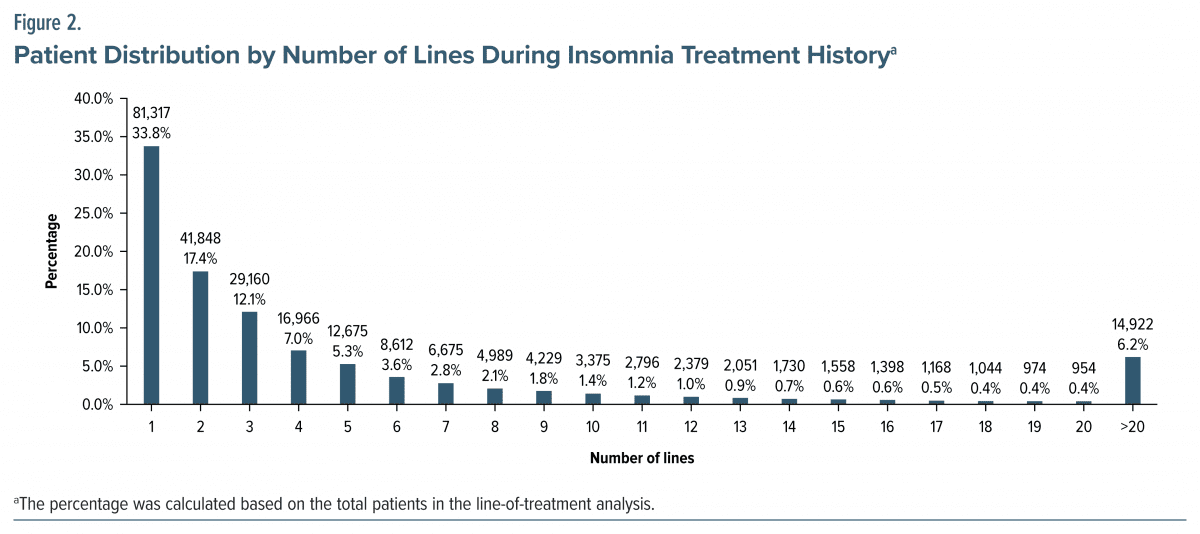
Number of treatment lines. The mean number of treatment lines was 6.4 (SD 14.3). Most patients (33.8%) received 1 line of treatment during the observation period, and 6.2% of patients had >20 lines of treatment over their entire insomnia intervention (Figure 2; Supplementary Table 5).
DISCUSSION
This retrospective observational real-world study examined insomnia treatments in a large, representative sample of Canadian patients. We described drug utilization, inappropriate drug usage, and lines of treatment across age and payer cohorts to understand insomnia intervention patterns from 2018 to 2020.
Canada has a mix of public and private insurance plans to cover prescription drugs in the outpatient setting. The public plans are offered by provincial and territorial governments to eligible residents based on age, income, and medical conditions.40,41
In this study, most patients were from the PDP database. Patients from the ODB database claimed multiple classes of insomnia medications more often and had a longer duration of medication use and higher treatment costs than private plan patients. This low capture of insomnia patients in public plan and difference in drug utilization between public and private patients could be explained by the restricted access to on label prescription interventions for insomnia in public plan. In Ontario, almost all BZD, antidepressants, and antipsychotic drugs are funded publicly, while Z-drugs and lemborexant are not. Zopiclone is only covered under the Exceptional Access Program (a program designed to facilitate patient access to drugs in Ontario).
There was a higher proportion of high polypharmacy patients (≥ 5 drug classes) in 2018 and 2019 compared to 2020. A lower polypharmacy in 2020 is likely a result of the COVID-19 pandemic, which reduced patient access to health care services and reduced medication prescriptions.42 More than 52% of patients exhibited high polypharmacy (≥5 drug classes), which can be associated with higher risks, eg, drug-drug interactions; therefore, it is important to appropriately manage treatment.32
Inappropriate medication use, specifically for BZD and Z-drugs in the elderly, has been studied in Canada and globally. A study in Manitoba reported that 15.6%–35.1% of patients with Z-drugs had long-term use (≥90–180 days after first prescription).43 Similarly, a study in United States reported 31.4% of patients with BZD long-term use (≥120 days).44 In our study, we observed >50% of patients with extended duration of BZD and Z-drugs, which is higher than other studies. This could be explained by the different definition of long term use of 30 days in this study. Overall, the study results indicate that the prevalence of inappropriate use among patients with insomnia is high in Canada, and clinical guidelines and product monograph indications are not being followed appropriately. It is therefore important to raise awareness of inappropriate use, properly follow clinical guidelines to improve the medication prescribing practices, and explore other avenues such as deprescribing BZD and Z-drug to further reduce the potential harms among senior patients with insomnia. It needs efforts from all stakeholders including patients, physicians, pharmacists, and nurse practitioners to take action.
In the lines of treatment analysis, 6.2% of patients had >20 lines during their insomnia intervention history, suggesting that a subgroup dissatisfied with current interventions cycled through limited medication options. This indicates a need for better clinical assessment of insomnia by leveraging appropriate assessment tools and investigating patients’ pattern of drug use to provide proper management of insomnia and new treatments.45–47
This study utilizes a large and representative patient population from the public and private databases of Canada, allowing for an overview of drug utilization, inappropriate usage of medications, and lines of treatment in both the adult and senior populations with insomnia in Canada. This is the first study, to our best knowledge, that assessed the real-world drug utilization among patients with insomnia in Canada and specifically generated real-world evidence of inappropriate medication usage for the management of insomnia.
This study has several limitations worth noting. First, this study did not analyze cash-paying patients or publicly funded prescriptions outside of Ontario. Second, diagnostic information is not included in the PDP and ODB claims databases; therefore, the indication of insomnia in this study was inferred based on the prescription of 2 medication classes (lemborexant and Z-drugs) approved specifically for insomnia. The insomnia cases in Canada may be underestimated considering patients with insomnia may not take lemborexant and/or Z-drugs due to limited access or may choose other on-label drugs such as doxepin (3 mg or 6 mg) or some BZD drugs (flurazepam, nitrazepam, triazolam, and temazepam) as well as off-label options (BZD drugs and antidepressants or antipsychotic drugs). Further, this study followed a restricted and conservative approach (30-day consecutive use instead of 7–14 days, as per the product monograph) and did not include other interventions, such as alcohol and over-the-counter drugs. Considering these limitations, the insomnia population selected in this study could potentially be an under-representation of the true size.
CONCLUSION
This study described the drug utilization and the inappropriate usage of insomnia medications despite published prescribing guidelines and label recommendations in Canada. Inappropriate usage of insomnia medications was causing a substantial health care burden in Canada, especially in senior patients. This study demonstrates that there is a high prevalence of inappropriate medication usage in insomnia, particularly in seniors. Overall, this study shows a need for better education to general practitioners and the public of current guidelines30–32 and more effective and safer treatment options as well as appropriate patient monitoring tools for physicians, particularly for seniors who may require long-term treatment.
Article Information
Published Online: September 11, 2024. https://doi.org/10.4088/JCP.23m15015
© 2024 Physicians Postgraduate Press, Inc.
Submitted: July 14, 2023; accepted July 9, 2024.
To Cite: Kamboj L, Ramos B, Haynes A, et al. A retrospective observational study to understand medication utilization and lines of treatment in patients with insomnia disorder. J Clin Psychiatry. 2024;85(4):23m15015.
Author Affiliations: Eisai Ltd, Mississauga, Canada (Kamboj, Ramos, Haynes, Sohi); IQVIA Solutions Canada, Inc, Kirkland, Canada (Yang, Ling, Barot, Millson); Division of Geriatric Psychiatry, Queen’s University, Providence Care Hospital, Kingston, Canada (Amanullah).
Corresponding Author: Laveena Kamboj, MSc, Eisai Ltd, 6925 Century Ave #701, Mississauga, ON L5N 7K2, Canada ([email protected]).
Author Contributions: All authors contributed to data analysis and interpretation. Kamboj, Ramos, Sohi, Yang, Ling, Barot, and Amanullah contributed to study conceptualization. Sohi, Yang, Ling, and Barot contributed to study design. Yang contributed to data collection. All authors contributed to drafting, editing, and critically revising the manuscript. All authors are accountable for the work and provided their agreement for submission to the journal and approval for publication.
Relevant Financial Relationships: Ms Kamboj, Dr Ramos, and Mr Haynes are employees of Eisai. Dr Sohi is a former employee of Eisai. Dr Yang, Mss Ling and Barot, and Mr Millson are employees of IQVIA Solutions Canada Inc and received study funding from Eisai. Dr Amanullah is affiliated with Providence Care Hospital and received funding for support on this study.
Funding/Support: This study was sponsored by Eisai Ltd, Canada.
Role of the Funders/Sponsors: Eisai was involved in the study design and preparation of the manuscript. IQVIA was involved in the study design and preparation of the manuscript.
Previous Presentation: The study was disseminated at the Choosing Wisely Canada 2022 Conference; May 25–26, 2022; virtual, as an abstract, and was presented at the Canadian Association for Population Therapeutics (CAPT) 2022 Conference; October 17–18, 2022; Toronto, Canada, as an abstract and poster.
Acknowledgments: The authors thank Oliver Sang, BSc (IQVIA Canada), for data analysis support and thank Gargi Pal, PhD; Shlok Kumar, MA; and Lakshman Puli, PhD (IQVIA, India), for medical writing and editorial support. Study analysis and medical writing assistance were provided by IQVIA Canada.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- There is limited information regarding real-world treatment patterns for insomnia patients in Canada.
- There is a high prevalence of inappropriate medication usage in insomnia, and a subset of patients received more than 20 lines of insomnia interventions, highlighting a need for more effective and safer treatment options.
References (47)

- Daley M, Morin CM, LeBlanc M, et al. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427–438. PubMed CrossRef
- Bollu PC, Kaur H. Sleep medicine: insomnia and sleep. Mo Med. 2019;116(1):68–75. PubMed
- Garland SN, Rowe H, Repa LM, et al. A decade’s difference: 10-year change in insomnia symptom prevalence in Canada depends on sociodemographics and health status. Sleep Health. 2018;4(2):160–165. PubMed CrossRef
- Morin CM, LeBlanc M, Bélanger L, et al. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56(9):540–548. PubMed CrossRef
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. PubMed CrossRef
- Laugsand LE, Vatten LJ, Platou C, et al. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124(19):2073–2081. PubMed CrossRef
- Sofi F, Cesari F, Casini A, et al. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64. PubMed CrossRef
- Asche CV, Joish VN, Camacho F, et al. The direct costs of untreated comorbid insomnia in a managed care population with major depressive disorder. Curr Med Res Opin. 2010;26(8):1843–1853. PubMed CrossRef
- Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268–275.e2. PubMed CrossRef
- Taddei-Allen P. Economic burden and managed care considerations for the treatment of insomnia. Am J Manag Care. 2020;26(4 suppl):S91–S96. PubMed CrossRef
- Anderson LH, Whitebird RR, Schultz J, et al. Healthcare utilization and costs in persons with insomnia in a managed care population. Am J Manag Care. 2014;20(5):e157–e165. PubMed
- Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. PubMed CrossRef
- Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329–336. PubMed CrossRef
- Gui Z, Wang YY, Li JX, et al. Prevalence of poor sleep quality in COVID-19 patients: a systematic review and meta-analysis. Front Psychiatry. 2023;14:1272812. PubMed CrossRef
- Morin CM, Bjorvatn B, Chung F, et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med. 2021;87:38–45. PubMed
- Hoang HTX, Yeung WF, Truong QTM, et al. Sleep quality among non-hospitalized COVID-19 survivors: a national cross-sectional study. Front Public Health. 2024;11:1281012. PubMed CrossRef
- Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255–262. PubMed
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. PubMed CrossRef
- Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. PubMed
- Health Canada, Product Information: DAYVIGO (LEMBORECANT). Accessed March 22, 2023. https://pdf.hres.ca/dpd_pm/00071351.PDF
- Health Canada. Product Information: QUVIVIQ (DARIDOREXANT). Accessed February 6, 2024. https://pdf.hres.ca/dpd_pm/00074121.PDF
- Health Canada, Product Information: BELSOMRA (SUVOREXANT). Accessed February 13, 2024. https://pdf.hres.ca/dpd_pm/00051352.PDF
- Lie JD, Tu KN, Shen DD, et al. Pharmacological treatment of insomnia. P T. 2015;40(11):759–771. PubMed
- McGee N, Proctor JL, Hart AM, et al. Reconsidering benzodiazepines and Z-drug prescriptions: responsible prescribing and deprescribing. J Nurse Pract. 2021;17(1):76–83.
- Food and Drug Administration. FDA Drug Safety Communication: FDA urges caution about withholding opioid addiction medications from patients taking benzodiazepines or CNS depressants: careful medication management can reduce risks. Food and Drug Administration. Accessed May 23, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safetycommunication-fda-urges-caution-about-withholding-opioid-addictionmedications
- Health Canada. Updates to safety labelling for benzodiazepines and benzodiazepine-like drugs. Accessed May 23, 2022. https://recalls-rappels.canada.ca/en/alert-recall/updates-safety-labelling-benzodiazepines-andbenzodiazepine-drugs.
- Lavergne R, Peterson S, Rudoler D, et al. The same, only different: using physician billing data from four provincial payment systems to describe family physician practice patterns in Canada. Int J Popul Data Sci. 2022;7(3):1849.
- Uthayakumar S, Tadrous M, Vigod SN, et al. The effects of COVID-19 on the dispensing rates of antidepressants and benzodiazepines in Canada. Depress Anxiety. 2022;39(2):156–162. PubMed
- Ontario drug policy research network on behalf of the ODPRN citizens’ panel. Characterizing prescription benzodiazepine use among community-dwelling residents of Ontario, Canada. Ontario Drug Policy Research Network; 2021.
- Pottie K, Thompson W, Davies S, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(5):339–351. PubMed
- Conn DK, Hogan DB, Amdam L, et al. Canadian guidelines on benzodiazepine receptor agonist use disorder among older adults title. Can Geriatr J. 2020;23(1):116–122. PubMed
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American geriatrics society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. PubMed CrossRef
- McMillan JM, Aitken E, Holroyd-Leduc JM. Management of insomnia and long term use of sedative-hypnotic drugs in older patients. CMAJ. 2013;185(17):1499–1505. PubMed CrossRef
- Scharner V, Hasieber L, Sönnichsen A, et al. Efficacy and safety of Z-substances in the management of insomnia in older adults: a systematic review for the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr. 2022;22(1):87. PubMed
- Kripke DF. What do hypnotics cost hospitals and healthcare? F1000Res. 2017;6:542. PubMed CrossRef
- Ontario Health. Check medication coverage. Accessed February 14, 2024. https://www.ontario.ca/check-medication-coverage/
- Patricia R, Cardoso R, Morra D, Vera N, et al. Clinical Evaluation of Interventions for the Management of Insomnia: A Review of Reviews. Canadian Agency for Drugs and Technologies in Health technology review; 2018:364. Accessed March 22, 2023. https://www.cadth.ca/sites/default/files/pdf/op0527_insomnia_clinical-evaluation-corrected.pdf.
- Product Monograph Including Patient Medication Information. Eisai Ltd; 2019. Accessed March 22, 2023. https://pdf.hres.ca/dpd_pm/00058592.PDF.
- Brandt J, Leong C. Benzodiazepines and Z-drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17(4):493–507. PubMed CrossRef
- Health Canada. Canada’s health care system. Accessed February 14, 2024. https://www.canada.ca/en/health-canada/services/canada-health-care-system.html
- Health Canada. Prescription drug insurance coverage. Accessed February 14, 2024. https://www.canada.ca/en/health-canada/services/health-caresystem/pharmaceuticals/access-insurance-coverage-prescriptionmedicines.html
- Aboulatta L, Peymani P, Vaccaro C, et al. Drug utilization patterns before and during COVID-19 pandemic in Manitoba, Canada: a population-based study. PLoS One. 2022;17(11):e0278072. PubMed CrossRef
- Brandt J, Janzen D, Alessi-Severini S, et al. Risk of long-term benzodiazepine and Z-drug use following the first prescription among community-dwelling adults with anxiety/mood and sleep disorders: a retrospective cohort study. BMJ Open. 2021;11(11):e046916. PubMed CrossRef
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. PubMed CrossRef
- Cheung JMY, Jarrin DC, Beaulieu-Bonneau S, et al. Patterns of concomitant prescription, over-the-counter and natural sleep aid use over a 12-month period: a population based study. Sleep. 2021;44(11):zsab141. PubMed CrossRef
- Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry. 2019;18(3):337–352. PubMed CrossRef
- Pillai V, Cheng P, Kalmbach DA, et al. Prevalence and predictors of prescription sleep aid use among individuals with DSM-5 insomnia: the role of hyperarousal. Sleep. 2016;39(4):825–832. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite