Insomnia is the most common and refractory complaint in Veterans with posttraumatic stress disorder (PTSD).1–3 Preliminary evidence suggests that pimavanserin, a selective 5-HT2A partial agonist/antagonist approved by the US Food and Drug Administration for treatment of Parkinson’s disease psychosis,4 may improve insomnia without producing daytime somnolence or addictive potential.5,6 Its relative affinity for 5-HT2A receptors and long half-life (~55 hours) represent novel approaches to insomnia treatment.7 Like other 5-HT2A antagonists, pimavanserin may enhance “deep” (N3) sleep7,8; this sleep stage is deficient in patients with PTSD.9 Accordingly, this open-label pilot study of pimavanserin describes the experience of 6 adult Veterans with active PTSD and chronic insomnia.
Methods
The study was approved by the Institutional Review Board at Baylor College of Medicine and the Research & Development program of the Michael E. DeBakey VA Medical Center (MEDVAMC) and registered on ClinicalTrials.gov (NCT04188392). See ClinicalTrials.gov for additional eligibility information and feasibility outcomes.10 Briefly, we recruited non-elderly, medically healthy Veterans with chronic insomnia disorder11 of at least moderate severity12 and current PTSD11,13 from clinics at MEDVAMC between December 19, 2019–March 20, 2020 (pandemic) and June 16, 2021–August 8, 2021. Informed consent was obtained from participants prior to any procedure. After initial screening, subjects completed an at-home actigraphy monitoring week (Actiwatch Spectrum Pro, Philips Respironics); a first polysomnogram (PSG) (Sleepware G3, Philips Respironics) to screen for confounding sleep disorders and mitigate the “first night effect”;14 and a second, baseline PSG. Subjects then received fixed dose pimavanserin 34 mg at bedtime for 6 weeks. Dosing and duration mimicked the pivotal trial of pimavanserin for Parkinson’s disease psychosis.4,5 Its half-life permitted bedtime instead of daily dosing. Evaluations occurred at weeks 3 and 6 in person and otherwise weekly via telephone. Treatment concluded with repeat actigraphy, PSG, and an exit visit. PSGs were performed and scored according to standard criteria.15 Actigraphy rest intervals were manually edited in conjunction with abbreviated sleep diaries completed by subjects.16 Subjective12,13,17,18 and objective measures were compared at the prespecified time points of baseline and week 6 with paired, 2-tailed t tests. A P value of < .05 was considered significant.
Results
The characteristics of the 6 subjects were mean age 35.33 ± 6.35 years; 2 (33.33%) females; and mean education of 14 ± 1.79 years. Two (33.33%) were Black or African American, 3 were White (50%), and 1 was “Other” (16.67%). Two (33.33%) were Hispanic or Latino. Three (50%) were Army, 1 (16.7%) Navy, and 2 (33.3%) Marine Veterans. Time since active duty ranged from 3.6 to 15.6 years. All index traumas were deployment-related. Four (66.67%) had comorbid depressive disorders. One (16.7%) was taking a selective serotonin reuptake inhibitor.
All subjects reported severe sleep initiation insomnia on a nightly basis per the Insomnia Severity Index12; 5 additionally had severe sleep maintenance insomnia. No subjects were treated for sleep disordered breathing. One subject had mild obstructive sleep apnea during the screening PSG.
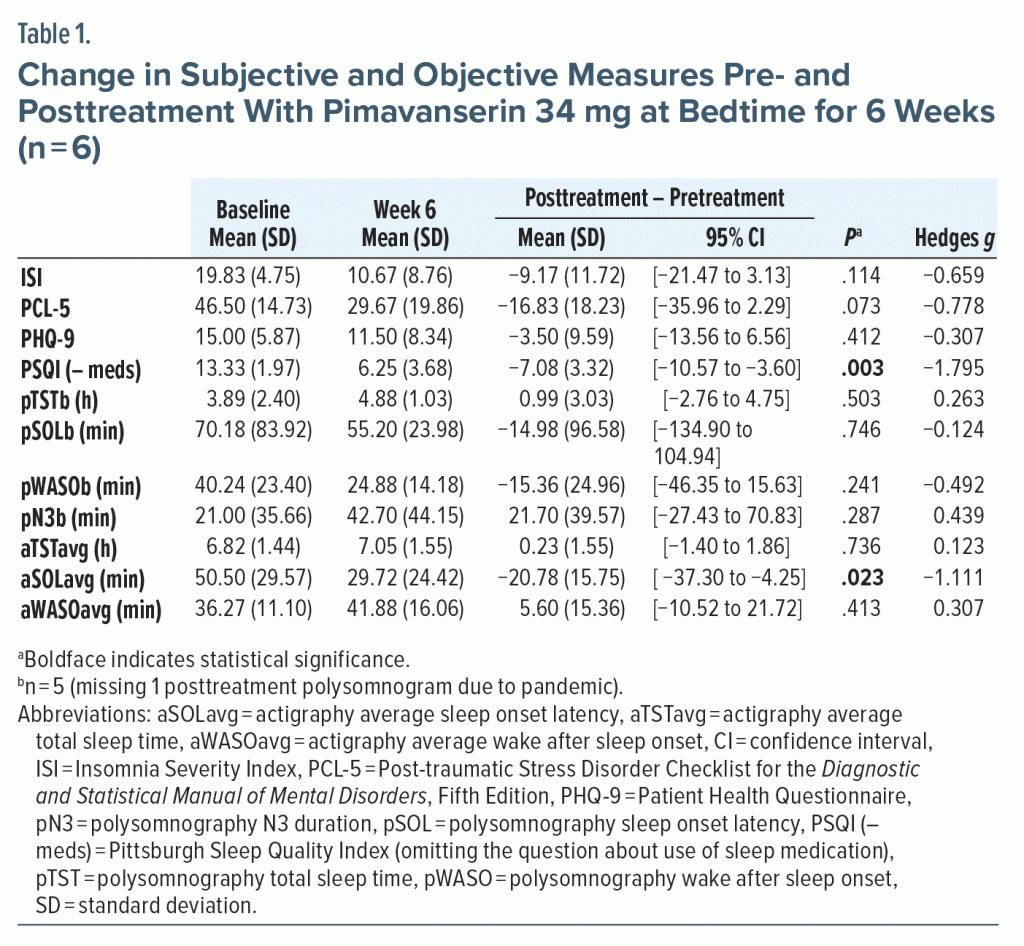
All subjects completed treatment. Subjective sleep quality significantly improved (see Table 1 and Supplementary Figure 1). PTSD trended toward improvement. Of the objective measures, only actigraphy derived sleep onset latency significantly decreased. The most frequent adverse event was mild sleepiness after the first dose (n = 2). No serious adverse events occurred.
After study completion, all subjects requested to continue the medication. Two subjects received non-formulary approval to resume pimavanserin 34 mg at bedtime due to previously unsuccessful medication trials for insomnia.
Discussion
This preliminary experience suggests pimavanserin may be well-tolerated at bedtime in patients with severe insomnia associated with PTSD. Patients reported subjective improvement in their insomnia symptoms and requested to continue the medication after the study. Randomized controlled trials are needed to test the efficacy and safety of pimavanserin against placebo. Future studies should also examine the mechanisms by which pimavanserin may influence sleep quality.
Article Information
Published Online: November 6, 2023. https://doi.org/10.4088/JCP.23br14992
© 2023 Physicians Postgraduate Press, Inc.
J Clin Psychiatry 2023;84(6):23br14992
Submitted: June 23, 2023; accepted September 7, 2023.
To Cite: Jones MB, Agrawal R, Sharafkhaneh A, et al. Pimavanserin 34 mg bedtime for the treatment of insomnia in 6 Veterans with posttraumatic stress disorder. J Clin Psychiatry. 2023;84(6):23br14992.
Author Affiliations: Mental Health Care Line, Michael E. DeBakey VA Medical Center, Houston, Texas (Jones, March, Jorge); Research Care Line, Michael E. DeBakey VA Medical Center, Houston, Texas (Jones, Bhatti); Beth K. and Stuart C. Yudofsky Division of Neuropsychiatry, Menninger Department of Psychiatry and Behavioral Sciences, Houston, Texas (Jones, Bhatti, March, Jorge); Medical Care Line, Michael E. DeBakey VA Medical Center, Houston, Texas (Agrawal, Sharafkhaneh); Section of Pulmonary, Critical Medicine, and Sleep Medicine, Department of Medicine, Baylor College of Medicine, Houston, Texas (Agrawal, Sharafkhaneh); Department of Biostatistics and Data Science, School of Public Health, University of Texas, Health Science Center at Houston, Houston (Li); Department of Neurology, Baylor College of Medicine, Houston, Texas (Marsh).
Corresponding Author: Melissa B. Jones, MD, 2002 Holcombe Blvd Rm 2B-122, 153 TBI, Houston, TX 77030 ([email protected]).
Relevant Financial Relationships: Drs Jones, Jorge, and Li receive study drug support for a VA CSR&D investigator-initiated trial (VA Career Development Award # IK2CX002363-01A1) from Acadia Pharmaceuticals. Dr Jorge receives study drug support for a VA Cooperative Studies Program trial (CSP 2018) from Pfizer Pharmaceuticals. Dr Marsh receives study drug support for a VA Cooperative Studies Program trial (CSP 2015) from Acadia Pharmaceuticals. The other authors have no conflicts of interest to disclose.
Funding/Support: This research was supported by the Center for Alzheimer’s and Neurodegenerative Diseases at Baylor College of Medicine, Houston, TX, and Career Development Award # IK2CX002363-01A1 from the U.S. Department of Veterans Affairs Clinical Science Research and Development (CSR&D) Service. Materials were provided by the Michael E. DeBakey VA Medical Center Seed & Bridge Award and Research Care Line, Houston, TX, and the Translational Research Center for Traumatic Brain Injury and Stress Disorders (TRACTS) (B9268-X) from the US Department of Veterans Affairs Rehabilitation Research and Development (RR&D) service.
Role of the Funders/Sponsors: The sponsors/supporters/funding agencies played no role in the conduct of the study; design, management, analysis, or interpretation of the data; and preparation, review or approval of the manuscript.
Disclaimer: The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Previous Presentation: This work was presented at the annual SLEEP meeting; June 5, 2023; Indianapolis, Indiana.
ORCID: Melissa B. Jones: https://orcid.org/0000-0002-3774-3356
Supplementary Material: Available at Psychiatrist.com.
References (18)

- Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929–933. PubMed CrossRef
- Larsen SE, Fleming CJE, Resick PA. Residual symptoms following empirically supported treatment for PTSD. Psychol Trauma. 2019;11(2):207–215. PubMed CrossRef
- Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51–e59. PubMed CrossRef
- Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540. PubMed CrossRef
- Patel N, LeWitt P, Neikrug AB, et al. Nighttime sleep and daytime sleepiness improved with pimavanserin during treatment of Parkinson’s disease psychosis. Clin Neuropharmacol. 2018;41(6):210–215. PubMed CrossRef
- Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2020;82(1):20m13425. PubMed CrossRef
- Ancoli-Israel S, Vanover KE, Weiner DM, et al. Pimavanserin tartrate, a 5-HT(2A) receptor inverse agonist, increases slow wave sleep as measured by polysomnography in healthy adult volunteers. Sleep Med. 2011;12(2):134–141. PubMed CrossRef
- Landolt HP, Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: mutual improvement of sleep, cognition and mood? Eur J Neurosci. 2009;29(9):1795–1809. PubMed CrossRef
- Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. PubMed CrossRef
- Pimavanserin for insomnia in veterans with posttraumatic stress disorder (PIP). ClinicalTrials.gov identifier: NCT04188392. Updated August 31, 2022. https://www.clinicaltrials.gov/study/NCT04188392?cond=pimavanserin%20insomnia&rank=2
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Publishing Inc; 2013.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. PubMed CrossRef
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379–1391. PubMed CrossRef
- Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric inpatients. Sleep. 1995;18(6):463–469. PubMed CrossRef
- Berry RBBR, Gamaldo CE, Hardling SM, et al. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; 2012.
- Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav Sleep Med. 2015;13(suppl 1):S4–S38. PubMed CrossRef
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. PubMed CrossRef
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite